Interactions of membrane-active peptides with thick, neutral, nonzwitterionic bilayers
- PMID: 16852806
- PMCID: PMC2532852
- DOI: 10.1021/jp050060x
Interactions of membrane-active peptides with thick, neutral, nonzwitterionic bilayers
Abstract
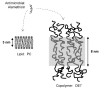
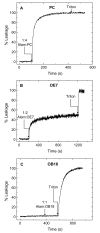
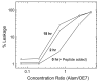
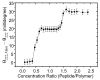
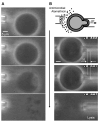
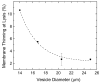
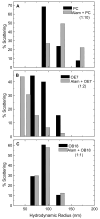
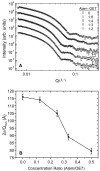
Alamethicin is a well-studied channel-forming peptide that has a prototypical amphipathic helix structure. It permeabilizes both microbial and mammalian cell membranes, causing loss of membrane polarization and leakage of endogenous contents. Antimicrobial peptide-lipid systems have been studied quite extensively and have led to significant advancements in membrane biophysics. These studies have been performed on lipid bilayers that are generally charged or zwitterionic and restricted to a thickness range of 3-5 nm. Bilayers of amphiphilic diblock copolymers are a relatively new class of membranes that can have significantly different physicochemical properties compared with those of lipid membranes. In particular, they can be made uncharged, nonzwitterionic, and much thicker than their lipid counterparts. In an effort to extend studies of membrane-protein interactions to these synthetic membranes, we have characterized the interactions of alamethicin and several other membrane-active peptides with diblock copolymer bilayers. We find that although alamethicin is too small to span the bilayer, the peptide interacts with, and ruptures, thick polymer membranes.
Figures


Similar articles
-
Alamethicin disrupts the cholesterol distribution in dimyristoyl phosphatidylcholine-cholesterol lipid bilayers.J Phys Chem B. 2014 Sep 25;118(38):11200-8. doi: 10.1021/jp504886u. Epub 2014 Sep 11. J Phys Chem B. 2014. PMID: 25210841
-
X-ray diffraction study of lipid bilayer membranes interacting with amphiphilic helical peptides: diphytanoyl phosphatidylcholine with alamethicin at low concentrations.Biophys J. 1995 Jun;68(6):2361-9. doi: 10.1016/S0006-3495(95)80418-2. Biophys J. 1995. PMID: 7647240 Free PMC article.
-
On the design of supramolecular assemblies made of peptides and lipid bilayers.J Pept Sci. 2014 Jul;20(7):526-36. doi: 10.1002/psc.2656. Epub 2014 Jun 7. J Pept Sci. 2014. PMID: 24909405
-
The mechanism of channel formation by alamethicin as viewed by molecular dynamics simulations.Novartis Found Symp. 1999;225:128-41; discussion 141-5. doi: 10.1002/9780470515716.ch9. Novartis Found Symp. 1999. PMID: 10472052 Review.
-
Simulation studies of the interaction of antimicrobial peptides and lipid bilayers.Biochim Biophys Acta. 1999 Dec 15;1462(1-2):185-200. doi: 10.1016/s0005-2736(99)00206-0. Biochim Biophys Acta. 1999. PMID: 10590308 Review.
Cited by
-
Possible Role of Bent Structure of Methylated Lithocholic Acid on Artificial and Plasma Membranes.Membranes (Basel). 2022 Oct 14;12(10):997. doi: 10.3390/membranes12100997. Membranes (Basel). 2022. PMID: 36295756 Free PMC article.
-
Simple surface functionalization of polymersomes using non-antibacterial peptide anchors.J Nanobiotechnology. 2016 Jun 22;14(1):48. doi: 10.1186/s12951-016-0205-x. J Nanobiotechnology. 2016. PMID: 27334900 Free PMC article.
-
Natural channel protein inserts and functions in a completely artificial, solid-supported bilayer membrane.Sci Rep. 2013;3:2196. doi: 10.1038/srep02196. Sci Rep. 2013. PMID: 23846807 Free PMC article.
-
Emerging Applications of Polymersomes in Delivery: from Molecular Dynamics to Shrinkage of Tumors.Prog Polym Sci. 2007 Aug 1;32(8-9):838-857. doi: 10.1016/j.progpolymsci.2007.05.011. Prog Polym Sci. 2007. PMID: 24692840 Free PMC article.
-
Melittin Induces Local Order Changes in Artificial and Biological Membranes as Revealed by Spectral Analysis of Laurdan Fluorescence.Toxins (Basel). 2020 Nov 8;12(11):705. doi: 10.3390/toxins12110705. Toxins (Basel). 2020. PMID: 33171598 Free PMC article.
References
-
- Discher BM, Won YY, Ege DS, Lee JC, Bates FS, Discher DE, Hammer DA. Science. 1999;284:1143. - PubMed
-
- Pata V, Ahmed F, Discher DE, Dan N. Langmuir. 2004;10:3888. - PubMed
-
- Meier W, Nardin C, Winterhalter M. Angew Chem Int Ed Engl. 2000;39:4599. - PubMed
-
- Duclohier H, Wroblewski H. J Membr Biol. 2001;184:1. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

