On possible pitfalls in ab initio quantum mechanics/molecular mechanics minimization approaches for studies of enzymatic reactions
- PMID: 16852982
- PMCID: PMC1514348
- DOI: 10.1021/jp0521757
On possible pitfalls in ab initio quantum mechanics/molecular mechanics minimization approaches for studies of enzymatic reactions
Abstract
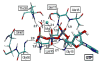
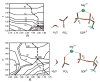
Reliable studies of enzymatic reactions by combined quantum mechanics/molecular mechanics (QM/MM) approaches, with an ab initio description of the quantum region, presents a major challenge to computational chemists. The main problem is the need for a very large computer time for the evaluation of the QM energy, which in turn makes it extremely challenging to perform proper configurational sampling. A seemingly reasonable alternative is to perform energy minimization studies of the type used in gas-phase ab initio studies. However, it is hard to see why such an approach should give reliable results in protein active sites. To examine the problems with energy minimization QM/MM approaches, we chose the hypothetical reaction of a metaphosphate ion with water in the Ras.GAP complex. This hypothetical reaction served as a simple benchmark reaction. The possible problems with the QM/MM minimization were explored by generating several protein configurations from long MD simulations and using energy minimization and scanning of the reaction coordinates to evaluate the corresponding potential energy surfaces of the reaction for each of these different protein configurations. Comparing these potential energy surfaces, we found major variations of the corresponding minima. Furthermore, the reaction energies and activation energies also varied significantly even for similar protein configurations. The specific coordination of a magnesium ion, present in the active center of the protein complex, turned out to influence the energetics of the reaction in a major way, where a direct coordination to the reactant leads to an increase of the activation energy by 17 kcal/mol. Apparently, using energy minimization to generate potential surfaces for an enzymatic reaction, while starting from a single protein structure, could lead to major errors in calculations of activation free energies and binding free energies. Thus we believe that extensive samplings of the configurational space of the protein are essential for meaningful determination of the energetics of enzymatic reactions. The possible relevance of our conclusion with regard to a recent study of the RasGAP reaction is discussed.
Figures
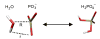




References
-
- Warshel A, Levitt M. Theoretical studies of enzymic reactions: dielectric, electrostatic and steric stabilization of the carbonium ion in the reaction of lysozyme. J Mol Biol. 1976;103:227. - PubMed
-
- Shurki A, Warshel A. Structure/Function Correlations of Protreins using MM, QM/MM and Related Approaches; Methods, Concepts, Pitfalls and Current Progress. Advances in Protein Chemistry. 2003;66:249. - PubMed
-
- Gao J. Hybrid quantum and molecular mechanical simulations: an alternative avenue to solvent effects in organic chemistry. Acc Chem Res. 1996;29:298.
-
- Bakowies D, Thiel W. Hybrid Models for Combined Quantum Mechanical and Molecular Approaches. J Phys Chem. 1996;100:10580.
-
- Field MJ, Bash PA, Karplus M. A Combined Quantum Mechanical and Molecular Mechanical Potential for Molecular Dynamics Simulations. J Comp Chem. 1990;11:700.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

