Correlation of free radical yields with strand break yields produced in plasmid DNA by the direct effect of ionizing radiation
- PMID: 16853159
- PMCID: PMC1847790
- DOI: 10.1021/jp0518409
Correlation of free radical yields with strand break yields produced in plasmid DNA by the direct effect of ionizing radiation
Abstract
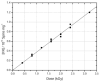
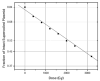
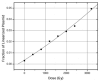
The purpose of this study was to determine how free radical formation (fr) correlates with single strand break (ssb) and double strand break (dsb) formation in DNA exposed to the direct effects of ionizing radiation. Chemical yields have been determined of (i) total radicals trapped on DNA at 4 K, G(Sigmafr), (ii) radicals trapped on the DNA sugar, Gsugar(fr), (iii) prompt single strand breaks, Gprompt(ssb), (iv) total single strand breaks, Gtotal(ssb), and (v) double strand breaks, G(dsb). These measurements make it possible, for the first time, to quantitatively test the premise that free radicals are the primary precursors to strand breaks. G(fr) were measured by EPR applied to films of pEC (10,810 bp) and pUC18 (2686 bp) plasmids hydrated to Gamma = 22 mol of water/nucleotide and X-irradiated at 4 K. Using these same samples warmed to room temperature, strand breaks were measured by gel electrophoresis. The respective values for pEC and pUC18 were G(fr) = 0.71 +/- 0.02 and 0.61 +/- 0.01 micromol/J, Gtotal(ssb) = 0.09 +/- 0.01 and 0.14 +/- 0.01 micromol/J, G(dsb) = 0.010 +/- 0.001 and 0.006 +/- 0.001 micromol/J, and Gtota)(ssb)/G(dsb) approximately 9 and approximately 20. Surprisingly, Gsugar(fr) approximately 0.06 mumol/J for pUC18 films, less than half of Gtotal(ssb). This indicates that a significant fraction of strand breaks are derived from precursors other than trapped DNA radicals. To explain this disparity, various mechanisms were considered, including one that entails two one-electron oxidations of a single deoxyribose carbon.
Figures


Similar articles
-
On the chemical yield of base lesions, strand breaks, and clustered damage generated in plasmid DNA by the direct effect of X rays.Radiat Res. 2007 Sep;168(3):357-66. doi: 10.1667/RR0964.1. Radiat Res. 2007. PMID: 17705639 Free PMC article.
-
An investigation into the mechanisms of DNA strand breakage by direct ionization of variably hydrated plasmid DNA.J Phys Chem B. 2006 Dec 28;110(51):26286-91. doi: 10.1021/jp065489i. J Phys Chem B. 2006. PMID: 17181287 Free PMC article.
-
Multiplicity of DNA single-strand breaks produced in pUC18 exposed to the direct effects of ionizing radiation.Radiat Res. 2008 Aug;170(2):156-62. doi: 10.1667/RR1277.1. Radiat Res. 2008. PMID: 18666814 Free PMC article.
-
Combination is the dominant free radical process initiated in DNA by ionizing radiation: an overview based on solid-state EPR studies.Int J Radiat Biol. 1994 Nov;66(5):491-7. doi: 10.1080/09553009414551511. Int J Radiat Biol. 1994. PMID: 7983436 Review.
-
The yield, processing, and biological consequences of clustered DNA damage induced by ionizing radiation.J Radiat Res. 2009 Jan;50(1):27-36. doi: 10.1269/jrr.08086. J Radiat Res. 2009. PMID: 19218779 Review.
Cited by
-
The role of hydration in the distribution of free radical trapping in directly ionized DNA.Radiat Res. 2006 Jul;166(1 Pt 1):1-8. doi: 10.1667/RR3585.1. Radiat Res. 2006. PMID: 16808596 Free PMC article.
-
On the chemical yield of base lesions, strand breaks, and clustered damage generated in plasmid DNA by the direct effect of X rays.Radiat Res. 2007 Sep;168(3):357-66. doi: 10.1667/RR0964.1. Radiat Res. 2007. PMID: 17705639 Free PMC article.
-
Mechanisms of strand break formation in DNA due to the direct effect of ionizing radiation: the dependency of free base release on the length of alternating CG oligodeoxynucleotides.J Phys Chem B. 2009 Jun 11;113(23):8183-91. doi: 10.1021/jp900803b. J Phys Chem B. 2009. PMID: 19492855 Free PMC article.
-
Mechanisms of direct radiation damage to DNA: the effect of base sequence on base end products.J Phys Chem B. 2011 Apr 28;115(16):4843-55. doi: 10.1021/jp200902h. Epub 2011 Apr 7. J Phys Chem B. 2011. PMID: 21473599 Free PMC article.
-
Mechanisms of direct radiation damage in DNA, based on a study of the yields of base damage, deoxyribose damage, and trapped radicals in d(GCACGCGTGC)(2).Radiat Res. 2007 Sep;168(3):367-81. doi: 10.1667/RR1058.1. Radiat Res. 2007. PMID: 17705640 Free PMC article.
References
-
- Spinks JWT, Woods RJ. Introduction to Radiation Chemistry. John Wiley & Sons; New York: 1990. p. 574.
-
- Sevilla MD, Becker D, Razskazovskii Y. Nukleonika. 1997;42:283–291.
-
- Krisch RE, Flick MB, Trumbore CN. Radiat Res. 1991;126:251–259. - PubMed
-
- Chapman JD. Biophysical models of mammalian cell inactivation by radiation. In: Meyn RE, Withers HR, editors. Radiation Biology in Cancer Research. Raven Press; New York: 1980. pp. 21–32.
-
- Prise KM. Spec Publ- R Soc Chem. 1997;204:111–116.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

