Incorporation of an intramolecular hydrogen-bonding motif in the side chain of 4-aminoquinolines enhances activity against drug-resistant P. falciparum
- PMID: 16854059
- PMCID: PMC1524878
- DOI: 10.1021/jm0600951
Incorporation of an intramolecular hydrogen-bonding motif in the side chain of 4-aminoquinolines enhances activity against drug-resistant P. falciparum
Erratum in
- J Med Chem. 2008 Feb 14;51(3):699
Abstract
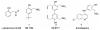
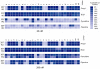
Previous data showing that several chloroquine analogues containing an intramolecular hydrogen-bonding motif were potent against multidrug-resistant P. falciparum led to the exploration of the importance of this motif. A series of 116 compounds containing four different alkyl linkers and various aromatic substitutions with hydrogen bond accepting capability was synthesized. The series showed broad potency against the drug-resistant W2 strain of P. falciparum. In particular, a novel series containing variations of the alpha-aminocresol motif gave eight compounds with IC50 values more potent than 5 nM against the W2 strain. Such simple modifications, significantly altering the pKa and sterics of the basic side chain in chloroquine analogues, may prove to be part of a strategy for overcoming the problem of worldwide resistance to affordable antimalarial drugs.
Figures


References
-
- Baird JK. Effectiveness of antimalarial drugs. N Engl J Med. 2005;352(15):1565–77. - PubMed
-
- Sachs J, Malaney P. The economic and social burden of malaria. Nature. 2002;415(6872):680–5. - PubMed
-
- Ridley RG, Hofheinz W, Matile H, Jaquet C, Dorn A, Masciadri R, Jolidon S, Richter WF, Guenzi A, Girometta MA, Urwyler H, Huber W, Thaithong S, Peters W. 4-aminoquinoline analogs of chloroquine with shortened side chains retain activity against chloroquine-resistant Plasmodium falciparum. Antimicrob Agents Chemother. 1996;40(8):1846–54. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

