Retinoic acid regulates the expression of photoreceptor transcription factor NRL
- PMID: 16854989
- PMCID: PMC1592579
- DOI: 10.1074/jbc.M605500200
Retinoic acid regulates the expression of photoreceptor transcription factor NRL
Abstract
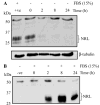
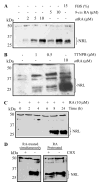
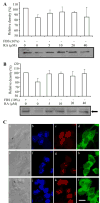
NRL (neural retina leucine zipper) is a key basic motif-leucine zipper (bZIP) transcription factor, which orchestrates rod photoreceptor differentiation by activating the expression of rod-specific genes. The deletion of Nrl in mice results in functional cones that are derived from rod precursors. However, signaling pathways modulating the expression or activity of NRL have not been elucidated. Here, we show that retinoic acid (RA), a diffusible factor implicated in rod development, activates the expression of NRL in serum-deprived Y79 human retinoblastoma cells and in primary cultures of rat and porcine photoreceptors. The effect of RA is mimicked by TTNPB, a RA receptor agonist, and requires new protein synthesis. DNaseI footprinting and electrophoretic mobility shift assays (EMSA) using bovine retinal nuclear extract demonstrate that RA response elements (RAREs) identified within the Nrl promoter bind to RA receptors. Furthermore, in transiently transfected Y79 and HEK293 cells the activity of Nrl-promoter driving a luciferase reporter gene is induced by RA, and this activation is mediated by RAREs. Our data suggest that signaling by RA via RA receptors regulates the expression of NRL, providing a framework for delineating early steps in photoreceptor cell fate determination.
Figures

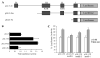
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

