TIP-1 has PDZ scaffold antagonist activity
- PMID: 16855024
- PMCID: PMC1635354
- DOI: 10.1091/mbc.e06-02-0129
TIP-1 has PDZ scaffold antagonist activity
Abstract
PDZ proteins usually contain multiple protein-protein interaction domains and act as molecular scaffolds that are important for the generation and maintenance of cell polarity and cell signaling. Here, we identify and characterize TIP-1 as an atypical PDZ protein that is composed almost entirely of a single PDZ domain and functions as a negative regulator of PDZ-based scaffolding. We found that TIP-1 competes with the basolateral membrane mLin-7/CASK complex for interaction with the potassium channel Kir 2.3 in model renal epithelia. Consequently, polarized plasma membrane expression of Kir 2.3 is disrupted resulting in pronounced endosomal targeting of the channel, similar to the phenotype observed for mutant Kir 2.3 channels lacking the PDZ-binding motif. TIP-1 is ubiquitously expressed, raising the possibility that TIP-1 may play a similar role in regulating the expression of other membrane proteins containing a type I PDZ ligand.
Figures




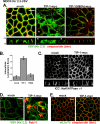
 ). Reporter activation is observed only in the presence of galactose. Mean ± SE; n = 3; * p ≤ 0.01; statistical significance as measured by ANOVA. (B) Three-dimensional structure of the TIP-1-binding pocket as modeled on the crystal structure of the related third PDZ domain of PSD-95 (PDB,1BFE; Doyle et al., 1996). Two residues, K20 and H90, predicted to interact with the PDZ ligand (blue) are shown in red. (C) GST pulldown assays using GST-Kir 2.3 C-terminus wild-type (WT) or a mutant GST-Kir 2.3 C-terminus lacking the PDZ interaction motif (ΔPDZ) and different purified recombinant His-tagged TIP-1 proteins (WT, wild-type TIP-1, and TIP-1-bearing K20A, H90A, or double K20A/H90A mutations). His-TIP-1 proteins specifically bound to the GST-Kir 2.3 proteins were detected by immunoblotting with anti-His antibodies (“pull-down”). In bottom panels, Ponceau S stain of GST-Kir 2.3 input and Coomassie stain of His-TIP-1 input are shown as loading controls. (D) The amount of each TIP-1 construct bound to GST-Kir 2.3 was assessed by densitometry, background subtracted, and analyzed relative to the WT TIP-1 band intensity. The mean ± SE relative densitometry of four pulldown experiments repeated in triplicate (p ≤ 0.01) is shown.
). Reporter activation is observed only in the presence of galactose. Mean ± SE; n = 3; * p ≤ 0.01; statistical significance as measured by ANOVA. (B) Three-dimensional structure of the TIP-1-binding pocket as modeled on the crystal structure of the related third PDZ domain of PSD-95 (PDB,1BFE; Doyle et al., 1996). Two residues, K20 and H90, predicted to interact with the PDZ ligand (blue) are shown in red. (C) GST pulldown assays using GST-Kir 2.3 C-terminus wild-type (WT) or a mutant GST-Kir 2.3 C-terminus lacking the PDZ interaction motif (ΔPDZ) and different purified recombinant His-tagged TIP-1 proteins (WT, wild-type TIP-1, and TIP-1-bearing K20A, H90A, or double K20A/H90A mutations). His-TIP-1 proteins specifically bound to the GST-Kir 2.3 proteins were detected by immunoblotting with anti-His antibodies (“pull-down”). In bottom panels, Ponceau S stain of GST-Kir 2.3 input and Coomassie stain of His-TIP-1 input are shown as loading controls. (D) The amount of each TIP-1 construct bound to GST-Kir 2.3 was assessed by densitometry, background subtracted, and analyzed relative to the WT TIP-1 band intensity. The mean ± SE relative densitometry of four pulldown experiments repeated in triplicate (p ≤ 0.01) is shown.



Similar articles
-
Lin-7 targets the Kir 2.3 channel on the basolateral membrane via a L27 domain interaction with CASK.Am J Physiol Cell Physiol. 2007 Dec;293(6):C1733-41. doi: 10.1152/ajpcell.00323.2007. Epub 2007 Oct 3. Am J Physiol Cell Physiol. 2007. PMID: 17913842
-
Basolateral membrane expression of the Kir 2.3 channel is coordinated by PDZ interaction with Lin-7/CASK complex.Am J Physiol Cell Physiol. 2002 Jan;282(1):C183-95. doi: 10.1152/ajpcell.00249.2001. Am J Physiol Cell Physiol. 2002. PMID: 11742811
-
Molecular mechanism of inward rectifier potassium channel 2.3 regulation by tax-interacting protein-1.J Mol Biol. 2009 Oct 2;392(4):967-76. doi: 10.1016/j.jmb.2009.07.060. Epub 2009 Jul 25. J Mol Biol. 2009. PMID: 19635485
-
PDZ proteins retain and regulate membrane transporters in polarized epithelial cell membranes.Am J Physiol Cell Physiol. 2005 Jan;288(1):C20-9. doi: 10.1152/ajpcell.00368.2004. Am J Physiol Cell Physiol. 2005. PMID: 15591244 Review.
-
Sending signals dynamically.Science. 2009 Apr 10;324(5924):198-203. doi: 10.1126/science.1169377. Science. 2009. PMID: 19359576 Free PMC article. Review.
Cited by
-
Expression of TIP-1 confers radioresistance of malignant glioma cells.PLoS One. 2012;7(9):e45402. doi: 10.1371/journal.pone.0045402. Epub 2012 Sep 17. PLoS One. 2012. PMID: 23028987 Free PMC article.
-
Specificity and promiscuity in human glutaminase interacting protein recognition: insight from the binding of the internal and C-terminal motif.Biochemistry. 2012 Sep 4;51(35):6950-60. doi: 10.1021/bi3008033. Epub 2012 Aug 21. Biochemistry. 2012. PMID: 22876914 Free PMC article.
-
Regulation of potassium channel trafficking in the distal nephron.Curr Opin Nephrol Hypertens. 2013 Sep;22(5):559-65. doi: 10.1097/MNH.0b013e328363ff76. Curr Opin Nephrol Hypertens. 2013. PMID: 23892700 Free PMC article. Review.
-
Aberrant nuclear localization of EBP50 promotes colorectal carcinogenesis in xenotransplanted mice by modulating TCF-1 and β-catenin interactions.J Clin Invest. 2012 May;122(5):1881-94. doi: 10.1172/JCI45661. Epub 2012 Apr 2. J Clin Invest. 2012. PMID: 22466651 Free PMC article.
-
PDZ Domain Recognition: Insight from Human Tax-Interacting Protein 1 (TIP-1) Interaction with Target Proteins.Biology (Basel). 2015 Feb 5;4(1):88-103. doi: 10.3390/biology4010088. Biology (Basel). 2015. PMID: 25665168 Free PMC article. Review.
References
-
- Ango F., Prezeau L., Muller T., Tu J. C., Xiao B., Worley P. F., Pin J. P., Bockaert J., Fagni L. Agonist-independent activation of metabotropic glutamate receptors by the intracellular protein Homer. Nature. 2001;411:962–965. - PubMed
-
- Borg J. P., Straight S. W., Kaech S. M., de Taddeo-Borg M., Kroon D. E., Karnak D., Turner R. S., Kim S. K., Margolis B. Identification of an evolutionarily conserved heterotrimeric protein complex involved in protein targeting. J. Biol. Chem. 1998a;273:31633–31636. - PubMed
-
- Brone B., Eggermont J. PDZ proteins retain and regulate membrane transporters in polarized epithelial cell membranes. Am. J. Physiol. Cell Physiol. 2005;288:C20–C29. - PubMed
-
- Butz S., Okamoto M., Sudhof T. C. A. tripartite protein complex with the potential to couple synaptic vesicle exocytosis to cell adhesion in brain. Cell. 1998;94:773–782. - PubMed
-
- Cao T. T., Deacon H. W., Reczek D., Bretscher A., von Zastrow M. A kinase-regulated PDZ-domain interaction controls endocytic sorting of the β2-adrenergic receptor. Nature. 1999;401:286–290. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

