A simple account of cyclopean edge responses in macaque v2
- PMID: 16855086
- PMCID: PMC6674290
- DOI: 10.1523/JNEUROSCI.5308-05.2006
A simple account of cyclopean edge responses in macaque v2
Abstract
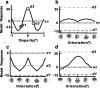
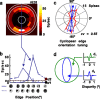
It has been shown recently that neurons in V2 respond selectively to the edges of figures defined only by disparity (cyclopean edges). These responses are orientation selective, often preferring similar orientations for cyclopean and luminance contours, suggesting that they may support a cue-invariant representation of contours. Here, we investigate the extent to which processing of purely local visual information (in the vicinity of the receptive field) might explain such results, using the most impoverished stimulus possible containing a cyclopean edge (a circular patch of random dots divided into two regions by a single edge). Many V2 cells responded better to the cyclopean edge than to uniform disparities, and most of these were at least broadly selective for the orientation of the cyclopean edge. Two characteristics argue against a cue-invariant contour representation: (1) the cyclopean edge response was frequently abolished by small changes to the component disparities; and (2) although V2 cells frequently responded to both signs of a cyclopean edge (defined by which side of the edge is in front), they did so at different edge locations. These characteristics are consistent with a simple feedforward scheme in which V2 neurons receive inputs from several V1 subunits with different disparity selectivity. We also found a correlation between the preferred orientations for cyclopean edges and contrast stimuli, suggesting that this feedforward wiring is not random. These characteristics suggest that V2 responses to cyclopean edges may be useful in supporting a cue-invariant contour representation higher in the visual pathway.
Figures











References
-
- Adelson EH, Bergen JR (1985). Spatiotemporal energy models for the perception of motion. J Opt Soc Am A 2:284–299. - PubMed
-
- Boynton GM, Hegde J (2004). Visual cortex: the continuing puzzle of area V2. Curr Biol 14:R523–R524. - PubMed
-
- Ferster D, Miller KD (2000). Neural mechanisms of orientation selectivity in the visual cortex. Annu Rev Neurosci 23:441–471. - PubMed
-
- Fleet DJ, Wagner H, Heeger DJ (1996). Neural encoding of binocular disparity: energy models, position shifts and phase shifts. Vision Res 36:1839–1857. - PubMed
-
- Hawken MJ, Shapley RM, Grosof DH (1996). Temporal-frequency selectivity in monkey visual cortex. Vis Neurosci 13:477–492. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
