Central roles of the roof plate in telencephalic development and holoprosencephaly
- PMID: 16855091
- PMCID: PMC6674267
- DOI: 10.1523/JNEUROSCI.0714-06.2006
Central roles of the roof plate in telencephalic development and holoprosencephaly
Abstract
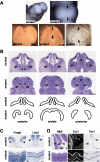
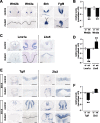
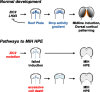
The roof plate is a well known signaling center in CNS development, but its roles in the developing telencephalon and the common holoprosencephaly (HPE) malformation have been uncertain. Using cellular ablations in mice, we show that roof plate cell loss causes failed midline induction and HPE in the dorsal telencephalon. This morphologic phenotype is accompanied by selective deficits in midline gene expression and a reduced activity gradient for bone morphogenetic proteins (Bmps), the major signals produced by the roof plate. In dissociated cells and mutant explants, exogenous Bmp4 is sufficient to mimic roof plate selectivity in midline gene regulation and to rescue roof plate-dependent midline patterning. Previously unrecognized neuroanatomical defects predicted by the mouse model are then confirmed in human HPE patients. These findings establish selective roles for roof plate-dependent Bmp signaling in dorsal telencephalic patterning and HPE and define novel candidate genes for the human disorder.
Figures




References
-
- Anderson RM, Lawrence AR, Stottmann RW, Bachiller D, Klingensmith J (2002). Chordin and noggin promote organizing centers of forebrain development in the mouse. Development 129:4975–4987. - PubMed
-
- Ashe HL, Briscoe J (2006). The interpretation of morphogen gradients. Development 133:385–394. - PubMed
-
- Bachiller D, Klingensmith J, Kemp C, Belo JA, Anderson RM, May SR, McMahon JA, McMahon AP, Harland RM, Rossant J, De Robertis EM (2000). The organizer factors Chordin and Noggin are required for mouse forebrain development. Nature 403:658–661. - PubMed
-
- Bishop KM, Goudreau G, O’Leary DD (2000). Regulation of area identity in the mammalian neocortex by Emx2 and Pax6. Science 288:344–349. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
