The glutamate-aspartate transporter GLAST mediates glutamate uptake at inner hair cell afferent synapses in the mammalian cochlea
- PMID: 16855093
- PMCID: PMC6674291
- DOI: 10.1523/JNEUROSCI.1545-06.2006
The glutamate-aspartate transporter GLAST mediates glutamate uptake at inner hair cell afferent synapses in the mammalian cochlea
Abstract
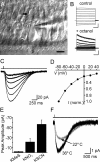
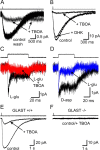
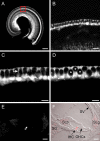
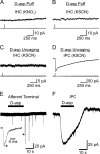
Ribbon synapses formed between inner hair cells (IHCs) and afferent dendrites in the mammalian cochlea can sustain high rates of release, placing strong demands on glutamate clearance mechanisms. To investigate the role of transporters in glutamate removal at these synapses, we made whole-cell recordings from IHCs, afferent dendrites, and glial cells adjacent to IHCs [inner phalangeal cells (IPCs)] in whole-mount preparations of rat organ of Corti. Focal application of the transporter substrate D-aspartate elicited inward currents in IPCs, which were larger in the presence of anions that permeate the transporter-associated anion channel and blocked by the transporter antagonist D,L-threo-beta-benzyloxyaspartate. These currents were produced by glutamate-aspartate transporters (GLAST) (excitatory amino acid transporter 1) because they were weakly inhibited by dihydrokainate, an antagonist of glutamate transporter-1 (excitatory amino acid transporter 2) and were absent from IPCs in GLAST-/- cochleas. Furthermore, D-aspartate-induced currents in outside-out patches from IPCs exhibited larger steady-state currents than responses elicited by L-glutamate, a prominent feature of GLAST, and examination of cochlea from GLAST-Discosoma red (DsRed) promoter reporter mice revealed that DsRed expression was restricted to IPCs and other supporting cells surrounding IHCs. Saturation of transporters by photolysis of caged D-aspartate failed to elicit transporter currents in IHCs, as did local application of D-aspartate to afferent terminals, indicating that neither presynaptic nor postsynaptic membranes are major sites for glutamate removal. These data indicate that GLAST in supporting cells is responsible for transmitter uptake at IHC afferent synapses.
Figures
Similar articles
-
Distribution of the glutamate/aspartate transporter GLAST in relation to the afferent synapses of outer hair cells in the guinea pig cochlea.J Assoc Res Otolaryngol. 2002 Sep;3(3):234-47. doi: 10.1007/s101620010064. Epub 2001 Dec 21. J Assoc Res Otolaryngol. 2002. PMID: 12382100 Free PMC article.
-
Identification of a glutamate/aspartate transporter in the rat cochlea.Hear Res. 1994 Aug;78(2):235-42. doi: 10.1016/0378-5955(94)90029-9. Hear Res. 1994. PMID: 7527019
-
Glutamate transporters in the guinea-pig cochlea: partial mRNA sequences, cellular expression and functional implications.Eur J Neurosci. 2003 Jan;17(1):83-92. doi: 10.1046/j.1460-9568.2003.02429.x. Eur J Neurosci. 2003. PMID: 12534971
-
Molecular organization of a type of peripheral glutamate synapse: the afferent synapses of hair cells in the inner ear.Prog Neurobiol. 1998 Feb;54(2):127-48. doi: 10.1016/s0301-0082(97)00054-3. Prog Neurobiol. 1998. PMID: 9481795 Review.
-
[Role of glutamate transporters in excitatory synapses in cerebellar Purkinje cells].Brain Nerve. 2007 Jul;59(7):669-76. Brain Nerve. 2007. PMID: 17663137 Review. Japanese.
Cited by
-
Circadian vulnerability of cisplatin-induced ototoxicity in the cochlea.FASEB J. 2020 Oct;34(10):13978-13992. doi: 10.1096/fj.202001236R. Epub 2020 Aug 24. FASEB J. 2020. PMID: 32840016 Free PMC article.
-
Expression of Atoh1, Gfi1, and Pou4f3 in the mature cochlea reprograms nonsensory cells into hair cells.Proc Natl Acad Sci U S A. 2024 Jan 30;121(5):e2304680121. doi: 10.1073/pnas.2304680121. Epub 2024 Jan 24. Proc Natl Acad Sci U S A. 2024. PMID: 38266052 Free PMC article.
-
Synaptic hyperexcitability of cytomegalic pyramidal neurons contributes to epileptogenesis in tuberous sclerosis complex.Cell Rep. 2022 Jul 19;40(3):111085. doi: 10.1016/j.celrep.2022.111085. Cell Rep. 2022. PMID: 35858542 Free PMC article.
-
Strategies for analyzing neuronal progenitor development and neuronal migration in the developing cerebral cortex.Cereb Cortex. 2011 Jul;21(7):1465-74. doi: 10.1093/cercor/bhq197. Epub 2010 Nov 15. Cereb Cortex. 2011. PMID: 21078821 Free PMC article. Review.
-
In vivo generation of immature inner hair cells in neonatal mouse cochleae by ectopic Atoh1 expression.PLoS One. 2014 Feb 20;9(2):e89377. doi: 10.1371/journal.pone.0089377. eCollection 2014. PLoS One. 2014. PMID: 24586731 Free PMC article.
References
-
- Basile AS, Huang JM, Xie C, Webster D, Berlin C, Skolnick P (1996). N-methyl-d-aspartate antagonists limit aminoglycoside antibiotic-induced hearing loss. Nat Med 2:1338–1343. - PubMed
-
- Bergles DE, Jahr CE (1997). Synaptic activation of glutamate transporters in hippocampal astrocytes. Neuron 19:1297–1308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
