Beta1-integrin signaling mediates premyelinating oligodendrocyte survival but is not required for CNS myelination and remyelination
- PMID: 16855094
- PMCID: PMC6674273
- DOI: 10.1523/JNEUROSCI.0444-06.2006
Beta1-integrin signaling mediates premyelinating oligodendrocyte survival but is not required for CNS myelination and remyelination
Abstract
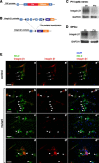
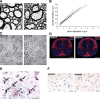
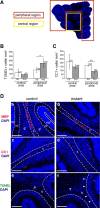
Previous reports, including transplantation experiments using dominant-negative inhibition of beta1-integrin signaling in oligodendrocyte progenitor cells, suggested that beta1-integrin signaling is required for myelination. Here, we test this hypothesis using conditional ablation of the beta1-integrin gene in oligodendroglial cells during the development of the CNS. This approach allowed us to study oligodendroglial beta1-integrin signaling in the physiological environment of the CNS, circumventing the potential drawbacks of a dominant-negative approach. We found that beta1-integrin signaling has a much more limited role than previously expected. Although it was involved in stage-specific oligodendrocyte cell survival, beta1-integrin signaling was not required for axon ensheathment and myelination per se. We also found that, in the spinal cord, remyelination occurred normally in the absence of beta1-integrin. We conclude that, although beta1-integrin may still contribute to other aspects of oligodendrocyte biology, it is not essential for myelination and remyelination in the CNS.
Figures



References
-
- Arnett HA, Fancy SP, Alberta JA, Zhao C, Plant SR, Kaing S, Raine CS, Rowitch DH, Franklin RJ, Stiles CD (2004). bHLH transcription factor Olig1 is required to repair demyelinated lesions in the CNS. Science 306:2111–2115. - PubMed
-
- Blaschuk KL, Frost EE, ffrench-Constant C (2000). The regulation of proliferation and differentiation in oligodendrocyte progenitor cells by alphaV integrins. Development 127:1961–1969. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
