Complex spike activity of purkinje cells in the oculomotor vermis during behavioral adaptation of monkey saccades
- PMID: 16855102
- PMCID: PMC6674272
- DOI: 10.1523/JNEUROSCI.4658-05.2006
Complex spike activity of purkinje cells in the oculomotor vermis during behavioral adaptation of monkey saccades
Abstract
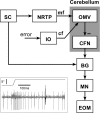
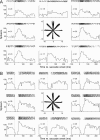
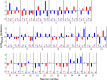
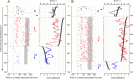
Throughout life, the oculomotor system can correct itself when saccadic eye movements become inaccurate. This adaptation mechanism can be engaged in the laboratory by displacing the target when the saccade toward it is in flight. Forward and backward target displacements cause gradual increases and decreases in saccade amplitude, respectively. Equipped with this paradigm, we asked whether Purkinje cells (P-cells) in the vermis of the oculomotor cerebellum, lobules VIc and VII, changed their complex spike (CS) discharge during the behavioral adaptation of horizontal saccades. We tested the hypothesis that CS activity would change only when a targeting saccade caused an error in eye position relative to the target, i.e., during the error interval between the primary and corrective saccades. We examined only those P-cells whose simple spike activity exhibited either a burst or pause with saccades in several directions. Approximately 80% of such P-cells exhibited an increase in CS activity during the error interval when the adaptation paradigm imposed horizontal eye-position errors in one direction and a decrease in activity for errors in the other. As adaptation progressed and errors were reduced, there was no consistent change in the CS activity. These data suggest that the CS activity of P-cells in the oculomotor vermis signals the direction but not the magnitude of eye-position error during saccade adaptation. Our results are consistent with cerebellar learning models that have been proposed to explain adaptation of the vestibulo-ocular reflex so similar mechanisms may also underlie plasticity of this precision voluntary oculomotor behavior.
Figures



Similar articles
-
Discharge properties of Purkinje cells in the oculomotor vermis during visually guided saccades in the macaque monkey.J Neurophysiol. 1995 Nov;74(5):1828-40. doi: 10.1152/jn.1995.74.5.1828. J Neurophysiol. 1995. PMID: 8592177
-
Changes in simple spike activity of some Purkinje cells in the oculomotor vermis during saccade adaptation are appropriate to participate in motor learning.J Neurosci. 2010 Mar 10;30(10):3715-27. doi: 10.1523/JNEUROSCI.4953-09.2010. J Neurosci. 2010. PMID: 20220005 Free PMC article.
-
Complex spike activity in the oculomotor vermis of the cerebellum: a vectorial error signal for saccade motor learning?J Neurophysiol. 2008 Oct;100(4):1949-66. doi: 10.1152/jn.90526.2008. Epub 2008 Jul 23. J Neurophysiol. 2008. PMID: 18650308 Free PMC article.
-
Cerebellar control of saccadic eye movements: its neural mechanisms and pathways.Jpn J Physiol. 1991;41(3):351-68. doi: 10.2170/jjphysiol.41.351. Jpn J Physiol. 1991. PMID: 1960885 Review.
-
How cerebellar motor learning keeps saccades accurate.J Neurophysiol. 2019 Jun 1;121(6):2153-2162. doi: 10.1152/jn.00781.2018. Epub 2019 Apr 17. J Neurophysiol. 2019. PMID: 30995136 Free PMC article. Review.
Cited by
-
Impairment of saccade adaptation in a patient with a focal thalamic lesion.J Neurophysiol. 2015 Apr 1;113(7):2351-9. doi: 10.1152/jn.00744.2014. Epub 2015 Feb 4. J Neurophysiol. 2015. PMID: 25652924 Free PMC article.
-
Saccade adaptation as a model of learning in voluntary movements.Exp Brain Res. 2010 Jul;204(2):145-62. doi: 10.1007/s00221-010-2314-3. Epub 2010 Jun 11. Exp Brain Res. 2010. PMID: 20544185 Review.
-
Encoding of error and learning to correct that error by the Purkinje cells of the cerebellum.Nat Neurosci. 2018 May;21(5):736-743. doi: 10.1038/s41593-018-0136-y. Epub 2018 Apr 16. Nat Neurosci. 2018. PMID: 29662213 Free PMC article.
-
Cerebellar complex spikes multiplex complementary behavioral information.PLoS Biol. 2021 Sep 16;19(9):e3001400. doi: 10.1371/journal.pbio.3001400. eCollection 2021 Sep. PLoS Biol. 2021. PMID: 34529650 Free PMC article.
-
P-sort: an open-source software for cerebellar neurophysiology.J Neurophysiol. 2021 Oct 1;126(4):1055-1075. doi: 10.1152/jn.00172.2021. Epub 2021 Aug 25. J Neurophysiol. 2021. PMID: 34432996 Free PMC article.
References
-
- Albus J (1971). A theory of cerebellar function. Math Biosci 10:25–61.
-
- Bahill AT, Clark MR, Stark L (1975). Dynamic overshoot in saccadic eye movements is caused by neurological control signed reversals. Exp Neurol 48:107–122. - PubMed
-
- Catz N, Dicke PW, Thier P (2005). Cerebellar complex spike firing is suitable to induce as well as to stabilize motor learning. Curr Biol 15:2179–2189. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
