Analysis of the ataxia telangiectasia mutated-mediated DNA damage response in murine cerebellar neurons
- PMID: 16855104
- PMCID: PMC6674276
- DOI: 10.1523/JNEUROSCI.2055-06.2006
Analysis of the ataxia telangiectasia mutated-mediated DNA damage response in murine cerebellar neurons
Abstract
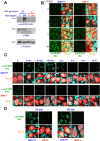
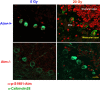
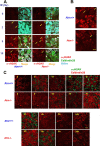
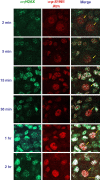
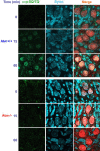
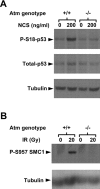
The DNA damage response is a network of signaling pathways that affects many aspects of cellular metabolism after the induction of DNA damage. The primary transducer of the cellular response to the double-strand break, a highly cytotoxic DNA lesion, is the nuclear protein kinase ataxia telangiectasia (A-T) mutated (ATM), which phosphorylates numerous effectors that play key roles in the damage response pathways. Loss or inactivation of ATM leads to A-T, an autosomal recessive disorder characterized by neuronal degeneration, particularly the loss of cerebellar granule and Purkinje cells, immunodeficiency, genomic instability, radiosensitivity, and cancer predisposition. The reason for the cerebellar degeneration in A-T is not clear. It has been ascribed by several investigators to cytoplasmic functions of ATM that may not be relevant to the DNA damage response. We set out to examine the subcellular localization of ATM and characterize the ATM-mediated damage response in mouse cerebellar neurons. We found that ATM is essentially nuclear in these cells and that various readouts of the ATM-mediated damage response are similar to those seen in commonly used cell lines. These include the autophosphorylation of ATM, which marks its activation, and phosphorylation of several of its downstream substrates. Importantly, all of these responses are detected in the nuclei of granule and Purkinje cells, suggesting that nuclear ATM functions in these cells similar to other cell types. These results support the notion that the cerebellar degeneration in A-T patients results from defective DNA damage response.
Figures

Similar articles
-
Nuclear ataxia-telangiectasia mutated (ATM) mediates the cellular response to DNA double strand breaks in human neuron-like cells.J Biol Chem. 2006 Jun 23;281(25):17482-17491. doi: 10.1074/jbc.M601895200. Epub 2006 Apr 20. J Biol Chem. 2006. PMID: 16627474
-
ATM localization and gene expression in the adult mouse eye.Mol Vis. 2009;15:393-416. Epub 2009 Feb 20. Mol Vis. 2009. PMID: 19234633 Free PMC article.
-
Contribution of the Atm protein to maintaining cellular homeostasis evidenced by continuous activation of the AP-1 pathway in Atm-deficient brains.J Biol Chem. 2003 Feb 28;278(9):6741-7. doi: 10.1074/jbc.M211168200. Epub 2002 Dec 19. J Biol Chem. 2003. PMID: 12496286
-
Atm and cellular response to DNA damage.Adv Exp Med Biol. 2005;570:457-76. doi: 10.1007/1-4020-3764-3_16. Adv Exp Med Biol. 2005. PMID: 18727511 Review. No abstract available.
-
ATM and the molecular pathogenesis of ataxia telangiectasia.Annu Rev Pathol. 2012;7:303-21. doi: 10.1146/annurev-pathol-011811-132509. Epub 2011 Oct 24. Annu Rev Pathol. 2012. PMID: 22035194 Review.
Cited by
-
In Cerebellar Atrophy of 12-Month-Old ATM-Null Mice, Transcriptome Upregulations Concern Most Neurotransmission and Neuropeptide Pathways, While Downregulations Affect Prominently Itpr1, Usp2 and Non-Coding RNA.Cells. 2023 Oct 3;12(19):2399. doi: 10.3390/cells12192399. Cells. 2023. PMID: 37830614 Free PMC article.
-
Ataxia telangiectasia: a review.Orphanet J Rare Dis. 2016 Nov 25;11(1):159. doi: 10.1186/s13023-016-0543-7. Orphanet J Rare Dis. 2016. PMID: 27884168 Free PMC article. Review.
-
Reduced synchronization persistence in neural networks derived from atm-deficient mice.Front Neurosci. 2011 Apr 4;5:46. doi: 10.3389/fnins.2011.00046. eCollection 2011. Front Neurosci. 2011. PMID: 21519382 Free PMC article.
-
Genome-Wide Screening in Human Embryonic Stem Cells Highlights the Hippo Signaling Pathway as Granting Synthetic Viability in ATM Deficiency.Cells. 2023 May 29;12(11):1503. doi: 10.3390/cells12111503. Cells. 2023. PMID: 37296624 Free PMC article.
-
Chronic Cough as a Genetic Neurological Disorder? Insights from Cerebellar Ataxia with Neuropathy and Vestibular Areflexia Syndrome (CANVAS).Lung. 2023 Dec;201(6):511-519. doi: 10.1007/s00408-023-00660-4. Epub 2023 Nov 18. Lung. 2023. PMID: 37979058 Free PMC article. Review.
References
-
- Abraham RT (2001). Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev 15:2177–2196. - PubMed
-
- Abraham RT (2004). PI 3-kinase related kinases: “big” players in stress-induced signaling pathways. DNA Repair (Amst) 3:883–887. - PubMed
-
- Abramova NE, Davies KJ, Crawford DR (2000). Polynucleotide degradation during early stage response to oxidative stress is specific to mitochondria. Free Radic Biol Med 28:281–288. - PubMed
-
- Andegeko Y, Moyal L, Mittelman L, Tsarfaty I, Shiloh Y, Rotman G (2001). Nuclear retention of ATM at sites of DNA double strand breaks. J Biol Chem 276:38224–38230. - PubMed
-
- Aylon Y, Kupiec M (2004). DSB repair: the yeast paradigm. DNA Repair (Amst) 3:797–815. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
