Outer membrane vesicle production by Escherichia coli is independent of membrane instability
- PMID: 16855227
- PMCID: PMC1540050
- DOI: 10.1128/JB.00498-06
Outer membrane vesicle production by Escherichia coli is independent of membrane instability
Abstract
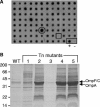
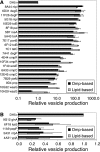
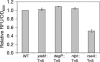
It has been long noted that gram-negative bacteria produce outer membrane vesicles, and recent data demonstrate that vesicles released by pathogenic strains can transmit virulence factors to host cells. However, the mechanism of vesicle release has remained undetermined. This genetic study addresses whether these structures are merely a result of membrane instability or are formed by a more directed process. To elucidate the regulatory mechanisms and physiological basis of vesiculation, we conducted a screen in Escherichia coli to identify gene disruptions that caused vesicle over- or underproduction. Only a few low-vesiculation mutants and no null mutants were recovered, suggesting that vesiculation may be a fundamental characteristic of gram-negative bacterial growth. Gene disruptions were identified that caused differences in vesicle production ranging from a 5-fold decrease to a 200-fold increase relative to wild-type levels. These disruptions included loci governing outer membrane components and peptidoglycan synthesis as well as the sigma(E) cell envelope stress response. Mutations causing vesicle overproduction did not result in upregulation of the ompC gene encoding a major outer membrane protein. Detergent sensitivity, leakiness, and growth characteristics of the novel vesiculation mutant strains did not correlate with vesiculation levels, demonstrating that vesicle production is not predictive of envelope instability.
Figures

References
-
- Alba, B. M., and C. A. Gross. 2004. Regulation of the Escherichia coli sigma-dependent envelope stress response. Mol. Microbiol. 52:613-619. - PubMed
-
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1998. Current protocols in molecular biology, vol. 1. John Wiley and Sons, Inc., Hoboken, N.J.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

