Identification and characterization of an Azotobacter vinelandii type I secretion system responsible for export of the AlgE-type mannuronan C-5-epimerases
- PMID: 16855245
- PMCID: PMC1540039
- DOI: 10.1128/JB.00236-06
Identification and characterization of an Azotobacter vinelandii type I secretion system responsible for export of the AlgE-type mannuronan C-5-epimerases
Abstract
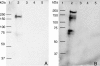
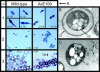
Alginate is a linear copolymer of beta-d-mannuronic acid and its C-5-epimer, alpha-l-guluronic acid. During biosynthesis, the polymer is first made as mannuronan, and various fractions of the monomers are then epimerized to guluronic acid by mannuronan C-5-epimerases. The Azotobacter vinelandii genome encodes a family of seven extracellular such epimerases (AlgE1 to AlgE7) which display motifs characteristic for proteins secreted via a type I pathway. Putative ATPase-binding cassette regions from the genome draft sequence of the A. vinelandii OP strain and experimentally verified type I transporters from other species were compared. This analysis led to the identification of one putative A. vinelandii type I system (eexDEF). The corresponding genes were individually disrupted in A. vinelandii strain E, and Western blot analysis using polyclonal antibodies against all AlgE epimerases showed that these proteins were present in wild-type culture supernatants but absent from the eex mutant supernatants. Consistent with this, the wild-type strain and the eex mutants produced alginate with about 20% guluronic acid and almost pure mannuronan (< or =2% guluronic acid), respectively. The A. vinelandii wild type is able to enter a particular desiccation-tolerant resting stage designated cyst. At this stage, the cells are surrounded by a rigid coat in which alginate is a major constituent. Such a coat was formed by wild-type cells in a particular growth medium but was missing in the eex mutants. These mutants were also found to be unable to survive desiccation. The reason for this is probably that continuous stretches of guluronic acid residues are needed for alginate gel formation to take place.
Figures




Similar articles
-
Cyclic di-GMP-Mediated Regulation of Extracellular Mannuronan C-5 Epimerases Is Essential for Cyst Formation in Azotobacter vinelandii.J Bacteriol. 2020 Nov 19;202(24):e00135-20. doi: 10.1128/JB.00135-20. Print 2020 Nov 19. J Bacteriol. 2020. PMID: 32989089 Free PMC article.
-
The Azotobacter vinelandii AlgE mannuronan C-5-epimerase family is essential for the in vivo control of alginate monomer composition and for functional cyst formation.Environ Microbiol. 2008 Jul;10(7):1760-70. doi: 10.1111/j.1462-2920.2008.01597.x. Epub 2008 Mar 28. Environ Microbiol. 2008. PMID: 18373676
-
Functional characterization of three Azotobacter chroococcum alginate-modifying enzymes related to the Azotobacter vinelandii AlgE mannuronan C-5-epimerase family.Sci Rep. 2020 Jul 27;10(1):12470. doi: 10.1038/s41598-020-68789-3. Sci Rep. 2020. PMID: 32719381 Free PMC article.
-
Hexuronyl C5-epimerases in alginate and glycosaminoglycan biosynthesis.Biochimie. 2001 Aug;83(8):819-30. doi: 10.1016/s0300-9084(01)01313-x. Biochimie. 2001. PMID: 11530215 Review.
-
Mannuronate C-5 epimerases and their use in alginate modification.Essays Biochem. 2023 Apr 18;67(3):615-627. doi: 10.1042/EBC20220151. Essays Biochem. 2023. PMID: 36876890 Review.
Cited by
-
Whole genome sequence analysis reveals the broad distribution of the RtxA type 1 secretion system and four novel putative type 1 secretion systems throughout the Legionella genus.PLoS One. 2020 Jan 14;15(1):e0223033. doi: 10.1371/journal.pone.0223033. eCollection 2020. PLoS One. 2020. PMID: 31935215 Free PMC article.
-
Structural and functional characterization of the R-modules in alginate C-5 epimerases AlgE4 and AlgE6 from Azotobacter vinelandii.J Biol Chem. 2014 Nov 7;289(45):31382-96. doi: 10.1074/jbc.M114.567008. Epub 2014 Sep 29. J Biol Chem. 2014. PMID: 25266718 Free PMC article.
-
Advances in alginate biosynthesis: regulation and production in Azotobacter vinelandii.Front Bioeng Biotechnol. 2025 Jul 31;13:1593893. doi: 10.3389/fbioe.2025.1593893. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40821665 Free PMC article. Review.
-
RpoS controls the expression and the transport of the AlgE1-7 epimerases in Azotobacter vinelandii.FEMS Microbiol Lett. 2018 Oct 1;365(19):fny210. doi: 10.1093/femsle/fny210. FEMS Microbiol Lett. 2018. PMID: 30169849 Free PMC article.
-
Increased c-di-GMP Levels Lead to the Production of Alginates of High Molecular Mass in Azotobacter vinelandii.J Bacteriol. 2020 Nov 19;202(24):e00134-20. doi: 10.1128/JB.00134-20. Print 2020 Nov 19. J Bacteriol. 2020. PMID: 32989088 Free PMC article.
References
-
- Bakkevig, K., H. Sletta, M. Gimmestad, R. Aune, H. Ertesvåg, K. Degnes, B. E. Christensen, T. E. Ellingsen, and S. Valla. 2005. Role of the Pseudomonas fluorescens alginate lyase (AlgL) in clearing the periplasm of alginates not exported to the extracellular environment. J. Bacteriol. 187:8375-8384. - PMC - PubMed
-
- Bendtsen, J. D., H. Nielsen, G. von Heijne, and S. Brunak. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340:783-795. - PubMed
-
- Bjerkan, T. M., C. L. Bender, H. Ertesvåg, F. Drabløs, M. K. Fakhr, L. A. Preston, G. Skjåk-Bræk, and S. Valla. 2004. The Pseudomonas syringae genome encodes a combined mannuronan C-5-epimerase and O-acetyl hydrolase, which strongly enhances the predicted gel-forming properties of alginates. J. Biol. Chem. 279:28920-28929. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

