Crystal structure of TDP-fucosamine acetyltransferase (WecD) from Escherichia coli, an enzyme required for enterobacterial common antigen synthesis
- PMID: 16855251
- PMCID: PMC1540030
- DOI: 10.1128/JB.00306-06
Crystal structure of TDP-fucosamine acetyltransferase (WecD) from Escherichia coli, an enzyme required for enterobacterial common antigen synthesis
Abstract
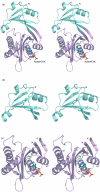
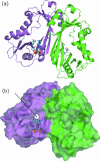
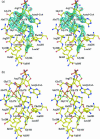
Enterobacterial common antigen (ECA) is a polysaccharide found on the outer membrane of virtually all gram-negative enteric bacteria and consists of three sugars, N-acetyl-d-glucosamine, N-acetyl-d-mannosaminuronic acid, and 4-acetamido-4,6-dideoxy-d-galactose, organized into trisaccharide repeating units having the sequence -->3)-alpha-d-Fuc4NAc-(1-->4)-beta-d-ManNAcA-(1-->4)-alpha-d-GlcNAc-(1-->. While the precise function of ECA is unknown, it has been linked to the resistance of Shiga-toxin-producing Escherichia coli (STEC) O157:H7 to organic acids and the resistance of Salmonella enterica to bile salts. The final step in the synthesis of 4-acetamido-4,6-dideoxy-d-galactose, the acetyl-coenzyme A (CoA)-dependent acetylation of the 4-amino group, is carried out by TDP-fucosamine acetyltransferase (WecD). We have determined the crystal structure of WecD in apo form at a 1.95-Angstrom resolution and bound to acetyl-CoA at a 1.66-Angstrom resolution. WecD is a dimeric enzyme, with each monomer adopting the GNAT N-acetyltransferase fold, common to a number of enzymes involved in acetylation of histones, aminoglycoside antibiotics, serotonin, and sugars. The crystal structure of WecD, however, represents the first structure of a GNAT family member that acts on nucleotide sugars. Based on this cocrystal structure, we have used flexible docking to generate a WecD-bound model of the acetyl-CoA-TDP-fucosamine tetrahedral intermediate, representing the structure during acetyl transfer. Our structural data show that WecD does not possess a residue that directly functions as a catalytic base, although Tyr208 is well positioned to function as a general acid by protonating the thiolate anion of coenzyme A.
Figures
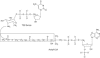

Similar articles
-
Identification of the structural gene for the TDP-Fuc4NAc:lipid II Fuc4NAc transferase involved in synthesis of enterobacterial common antigen in Escherichia coli K-12.J Bacteriol. 2001 Nov;183(22):6509-16. doi: 10.1128/JB.183.22.6509-6516.2001. J Bacteriol. 2001. PMID: 11673418 Free PMC article.
-
O acetylation of the enterobacterial common antigen polysaccharide is catalyzed by the product of the yiaH gene of Escherichia coli K-12.J Bacteriol. 2006 Nov;188(21):7542-50. doi: 10.1128/JB.00783-06. Epub 2006 Aug 25. J Bacteriol. 2006. PMID: 16936038 Free PMC article.
-
In vitro synthesis of a lipid-linked trisaccharide involved in synthesis of enterobacterial common antigen.J Bacteriol. 1989 Mar;171(3):1326-32. doi: 10.1128/jb.171.3.1326-1332.1989. J Bacteriol. 1989. PMID: 2493443 Free PMC article.
-
[The structure and significance of enterobacterial common antigen (ECA)].Postepy Hig Med Dosw (Online). 2015 Sep 8;69:1003-12. Postepy Hig Med Dosw (Online). 2015. PMID: 26400887 Review. Polish.
-
The serine acetyltransferase reaction: acetyl transfer from an acylpantothenyl donor to an alcohol.Arch Biochem Biophys. 2005 Jan 1;433(1):85-95. doi: 10.1016/j.abb.2004.08.014. Arch Biochem Biophys. 2005. PMID: 15581568 Review.
Cited by
-
In Silico Drug Repurposing Endorse Amprenavir, Darunavir and Saquinavir to Target Enzymes of Multidrug Resistant Uropathogenic E. Coli.Indian J Microbiol. 2024 Sep;64(3):1153-1214. doi: 10.1007/s12088-024-01282-x. Epub 2024 Apr 26. Indian J Microbiol. 2024. PMID: 39282172
-
Crystal structure of Helicobacter pylori pseudaminic acid biosynthesis N-acetyltransferase PseH: implications for substrate specificity and catalysis.PLoS One. 2015 Mar 17;10(3):e0115634. doi: 10.1371/journal.pone.0115634. eCollection 2015. PLoS One. 2015. PMID: 25781966 Free PMC article.
-
The structural biology of enzymes involved in natural product glycosylation.Nat Prod Rep. 2012 Oct;29(10):1201-37. doi: 10.1039/c2np20039b. Epub 2012 Jun 12. Nat Prod Rep. 2012. PMID: 22688446 Free PMC article. Review.
-
Protein Acetylation in Bacteria.Annu Rev Microbiol. 2019 Sep 8;73:111-132. doi: 10.1146/annurev-micro-020518-115526. Epub 2019 May 15. Annu Rev Microbiol. 2019. PMID: 31091420 Free PMC article. Review.
-
Small-Molecule Acetylation by GCN5-Related N-Acetyltransferases in Bacteria.Microbiol Mol Biol Rev. 2020 Apr 15;84(2):e00090-19. doi: 10.1128/MMBR.00090-19. Print 2020 May 20. Microbiol Mol Biol Rev. 2020. PMID: 32295819 Free PMC article. Review.
References
-
- Allard, S. T., W. W. Cleland, and H. M. Holden. 2004. High resolution X-ray structure of dTDP-glucose-4,6-dehydratase from Streptomyces venezuelae. J. Biol. Chem. 279:2211-2220. - PubMed
-
- Angus-Hill, M. L., R. N. Dutnall, S. T. Tafrov, R. Sternglanz, and V. Ramakrishnan. 1999. Crystal structure of the histone acetyltransferase Hpa2: a tetrameric member of the Gcn5-related N-acetyltransferase superfamily. J. Mol. Biol. 294:1311-1325. - PubMed
-
- Barr, K., and P. D. Rick. 1987. Biosynthesis of enterobacterial common antigen in Escherichia coli. In vitro synthesis of lipid-linked intermediates. J. Biol. Chem. 262:7142-7150. - PubMed
-
- Bayly, C. I., P. Cieplak, W. D. Cornell, and P. A. Kollman. 1993. A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: the RESP model. J. Phys. Chem. 97:10269-10280.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

