In vitro selection using a dual RNA library that allows primerless selection
- PMID: 16855281
- PMCID: PMC1524915
- DOI: 10.1093/nar/gkl463
In vitro selection using a dual RNA library that allows primerless selection
Abstract
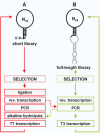
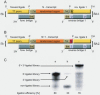
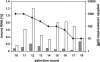
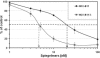
High affinity target-binding aptamers are identified from random oligonucleotide libraries by an in vitro selection process called Systematic Evolution of Ligands by EXponential enrichment (SELEX). Since the SELEX process includes a PCR amplification step the randomized region of the oligonucleotide libraries need to be flanked by two fixed primer binding sequences. These primer binding sites are often difficult to truncate because they may be necessary to maintain the structure of the aptamer or may even be part of the target binding motif. We designed a novel type of RNA library that carries fixed sequences which constrain the oligonucleotides into a partly double-stranded structure, thereby minimizing the risk that the primer binding sequences become part of the target-binding motif. Moreover, the specific design of the library including the use of tandem RNA Polymerase promoters allows the selection of oligonucleotides without any primer binding sequences. The library was used to select aptamers to the mirror-image peptide of ghrelin. Ghrelin is a potent stimulator of growth-hormone release and food intake. After selection, the identified aptamer sequences were directly synthesized in their mirror-image configuration. The final 44 nt-Spiegelmer, named NOX-B11-3, blocks ghrelin action in a cell culture assay displaying an IC50 of 4.5 nM at 37 degrees C.
Figures


Similar articles
-
Minimal primer and primer-free SELEX protocols for selection of aptamers from random DNA libraries.Biotechniques. 2008 Mar;44(3):351-60. doi: 10.2144/000112689. Biotechniques. 2008. PMID: 18361789
-
[Screening and characterization of DNA aptamers with hTNF-alpha binding and neutralizing activity].Sheng Wu Gong Cheng Xue Bao. 2003 Nov;19(6):730-3. Sheng Wu Gong Cheng Xue Bao. 2003. PMID: 15971588 Chinese.
-
Development of an automated in vitro selection protocol to obtain RNA-based aptamers: identification of a biostable substance P antagonist.Nucleic Acids Res. 2005 Mar 3;33(4):e45. doi: 10.1093/nar/gni044. Nucleic Acids Res. 2005. PMID: 15745995 Free PMC article.
-
SELEX--a (r)evolutionary method to generate high-affinity nucleic acid ligands.Biomol Eng. 2007 Oct;24(4):381-403. doi: 10.1016/j.bioeng.2007.06.001. Epub 2007 Jun 16. Biomol Eng. 2007. PMID: 17627883 Review.
-
Complex target SELEX.Acc Chem Res. 2008 Jan;41(1):130-8. doi: 10.1021/ar700142z. Acc Chem Res. 2008. PMID: 18193823 Review.
Cited by
-
An on-bead tailing/ligation approach for sequencing resin-bound RNA libraries.Nucleic Acids Res. 2012 May;40(9):e68. doi: 10.1093/nar/gks004. Epub 2012 Jan 31. Nucleic Acids Res. 2012. PMID: 22298510 Free PMC article.
-
Aptamers: A Review of Their Chemical Properties and Modifications for Therapeutic Application.Molecules. 2019 Nov 21;24(23):4229. doi: 10.3390/molecules24234229. Molecules. 2019. PMID: 31766318 Free PMC article. Review.
-
Key Aspects of Nucleic Acid Library Design for in Vitro Selection.Int J Mol Sci. 2018 Feb 5;19(2):470. doi: 10.3390/ijms19020470. Int J Mol Sci. 2018. PMID: 29401748 Free PMC article. Review.
-
RaptGen-Assisted Generation of an RNA/DNA Hybrid Aptamer against SARS-CoV-2 Spike Protein.Biochemistry. 2024 Apr 2;63(7):906-912. doi: 10.1021/acs.biochem.3c00596. Epub 2024 Mar 8. Biochemistry. 2024. PMID: 38457656 Free PMC article.
-
Cellular Delivery of RNA Nanoparticles.ACS Comb Sci. 2016 Sep 12;18(9):527-47. doi: 10.1021/acscombsci.6b00073. Epub 2016 Aug 26. ACS Comb Sci. 2016. PMID: 27509068 Free PMC article. Review.
References
-
- Ellington A.D., Szostak J.W. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346:818–822. - PubMed
-
- Tuerk C., Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249:505–510. - PubMed
-
- Thiel K. Oligo oligarchy-the surprisingly small world of aptamers. Nat. Biotechnol. 2004;22:649–651. - PubMed
-
- Yan A.C., Bell K.M., Breeden M.M., Ellington A.D. Aptamers: prospects in therapeutics and biomedicine. Front Biosci. 2005;10:1802–1827. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

