Defective splicing, disease and therapy: searching for master checkpoints in exon definition
- PMID: 16855287
- PMCID: PMC1524908
- DOI: 10.1093/nar/gkl498
Defective splicing, disease and therapy: searching for master checkpoints in exon definition
Abstract
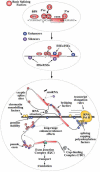
The number of aberrant splicing processes causing human disease is growing exponentially and many recent studies have uncovered some aspects of the unexpectedly complex network of interactions involved in these dysfunctions. As a consequence, our knowledge of the various cis- and trans-acting factors playing a role on both normal and aberrant splicing pathways has been enhanced greatly. However, the resulting information explosion has also uncovered the fact that many splicing systems are not easy to model. In fact we are still unable, with certainty, to predict the outcome of a given genomic variation. Nonetheless, in the midst of all this complexity some hard won lessons have been learned and in this survey we will focus on the importance of the wide sequence context when trying to understand why apparently similar mutations can give rise to different effects. The examples discussed in this summary will highlight the fine 'balance of power' that is often present between all the various regulatory elements that define exon boundaries. In the final part, we shall then discuss possible therapeutic targets and strategies to rescue genetic defects of complex splicing systems.
Figures






References
-
- Nissim-Rafinia M., Kerem B. The splicing machinery is a genetic modifier of disease severity. Trends Genet. 2005;21:480–483. - PubMed
-
- Caceres J.F., Kornblihtt A.R. Alternative splicing: multiple control mechanisms and involvement in human disease. Trends Genet. 2002;18:186–193. - PubMed
-
- Garcia-Blanco M.A., Baraniak A.P., Lasda E.L. Alternative splicing in disease and therapy. Nat. Biotechnol. 2004;22:535–546. - PubMed
-
- Cartegni L., Chew S.L., Krainer A.R. Listening to silence and understanding nonsense: exonic mutations that affect splicing. Nature Rev. Genet. 2002;3:285–298. - PubMed
-
- Faustino N.A., Cooper T.A. Pre-mRNA splicing and human disease. Genes Dev. 2003;17:419–437. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

