Long-acting beta2-agonists for poorly reversible chronic obstructive pulmonary disease
- PMID: 16855959
- PMCID: PMC11751769
- DOI: 10.1002/14651858.CD001104.pub2
Long-acting beta2-agonists for poorly reversible chronic obstructive pulmonary disease
Abstract
Background: Chronic obstructive pulmonary disease (COPD) is characterised by partially reversible airflow limitation. Many patients have little reversibility to short acting bronchodilators, but long acting bronchodilators are frequently advocated.
Objectives: To determine the effectiveness of long acting beta-2 adrenoceptor agonists (LABAs) in COPD patients demonstrating poor reversibility to short-acting bronchodilators.
Search strategy: The Cochrane Airways Group Specialised Register was searched ('all years' to 2005) along with the reference lists from identified randomised controlled trials (RCTs).
Selection criteria: All RCTs comparing inhaled LABAs (salmeterol or formoterol) with placebo in the treatment of patients with stable, poorly reversible COPD. Studies were a minimum of four weeks in duration.
Data collection and analysis: Two authors independently performed data extraction and study quality assessment. If we required additional data, we contacted authors and pharmaceutical companies sponsoring the identified RCTs.
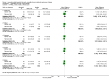
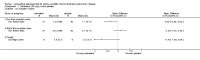
Main results: Twenty-three published and unpublished studies (6061 participants) were included in the review. There was a significant change in forced expiratory volume in 1 second (FEV1) in favour of salmeterol 50 mcg twice daily (BID) of 51 mls (95% confidence intervals (CI) 32 to 70), end of study morning peak expiratory flow (PEF) 14.89 L/min (95% CI 10.86 to 18.91). Supplemental short-acting bronchodilator usage was reduced by just under one puff per day. There were significant differences in the total, activity and impact domain scores of the St George's respiratory questionnaire in favour of salmeterol 50 mcg BID. Findings from other health status measurements and symptom scores were conflicting. There was no significant difference in exercise tolerance. The number of participants experiencing exacerbations was significantly reduced with salmeterol 50 mcg treatment compared with placebo (numbers needed to treat to benefit 21).
Authors' conclusions: This review shows that the treatment of patients with COPD with salmeterol 50 mcg produces modest increases in lung function. There were varying effects for other important outcomes such as health related quality of life or reduction in symptoms. However, there was a consistent reduction in exacerbations which may help people with COPD who suffer frequent deterioration of symptoms prompting healthcare utilisation. The strength of evidence for the use of salmeterol 100 mcg, formoterol 12 mcg, 18 mcg, 24 mcg was insufficient to provide clear indications for practice.
Conflict of interest statement
None known.
Figures
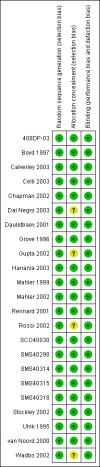

















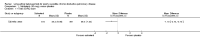


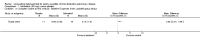








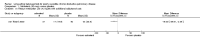
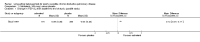






















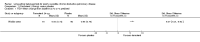
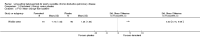









Update of
-
Long-acting beta2-agonists for chronic obstructive pulmonary disease patients with poorly reversible airflow limitation.Cochrane Database Syst Rev. 2002;(3):CD001104. doi: 10.1002/14651858.CD001104. Cochrane Database Syst Rev. 2002. Update in: Cochrane Database Syst Rev. 2006 Jul 19;(3):CD001104. doi: 10.1002/14651858.CD001104.pub2. PMID: 12137617 Updated.
Comment in
-
Review: long-acting beta2-adrenoceptor agonists are effective in poorly reversible chronic obstructive pulmonary disease.ACP J Club. 2007 Jan-Feb;146(1):18. ACP J Club. 2007. PMID: 17203938 No abstract available.
-
Review: long acting beta2 adrenoceptor agonists are effective in poorly reversible chronic obstructive pulmonary disease.Evid Based Med. 2007 Feb;12(1):12. doi: 10.1136/ebm.12.1.12. Evid Based Med. 2007. PMID: 17264260 No abstract available.
References
References to studies included in this review
408DP‐03 {published data only}
-
- 408D03. A multi‐center, randomized, double‐blind, parallel‐group, comparison of salmeterol xinafoate inhalation Rotadisk versus placebo in subjects with chronic obstructive pulmonary disease treated with current medications. GlaxoSmithKline Clinical Trial Register (http:ctr.gsk.co.uk) 2005.
Boyd 1997 {published and unpublished data}
-
- Boyd G, Morice AH, Pounsford JC, Siebert M, Peslis N, Crawford C. An evaluation of salmeterol in the treatment of chronic obstructive pulmonary disease (COPD). European Respiratory Journal 1997;10(4):815‐21. - PubMed
-
- Jones PW, Bosh TK. Quality of life changes in COPD patients treated with salmeterol. American Journal of Respiratory & Critical Care Medicine 1997;155:1283‐9. - PubMed
-
- SLGT28. A multicentre, randomised, double‐blind, parallel group study to compare the efficacy and safety of inhaled salmeterol xinafoate 50µg bd and inhaled salmeterol xinafoate 100µg bd with placebo, all administered via the metered‐dose inhaler, in the treatment of patients with chronic obstructive pulmonary disease. GlaxoSmithKline Clinical Trials Register (http:ctr.gsk.co.uk) 2005.
Calverley 2003 {published and unpublished data}
-
- Calverley P, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. The Lancet 2003;361(9356):449‐56. - PubMed
-
- Calverley PMA, Pauwels RA, Vestbo J, Jones PW, Pride NB, Gulsvik A, et al. Clinical improvements with salmeterol / fluticasone propionate combination in differing severities of COPD [A035] [Poster D50]. http://www.abstracts2view.com. 2003.
-
- Calverley PMA, Pauwels RA, Vestbo J, Jones PW, Pride NB, Gulsvik A, et al. Salmeterol/Fluticasone propionate combination for one year provides greater clinical benefit than its individual components [A98] [Poster: 306]. Proceedings of the 98th International American Thoracic Society Conference (http://www.abstracts‐on‐line.com/abstracts/ATS). 2002.
-
- Calverly PMA, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, et al. Safety of salmeterol/fluticasone propionate combination in the treatment of chronic obstructive pulmonary disease. European Respiratory Journal 2002;20(Suppl 38):242s.
-
- Hunjan MK, Chandler F. Numbers needed to treat (NNT) to avoid an exacerbation in patients with chronic obstructive pulmonary disease (COPD) using salmeterol/fluticasone propionate combination (SFC) and associated costs [Abstract]. American Thoracic Society 100th International Conference, May 21‐26. 2004:D22 Poster 503.
Celli 2003 {published data only}
-
- Celli B, Halpin D, Hepburn R, Byrne N, Keating E, Goldman M. Symptoms are an important outcome in chronic obstructive pulmonary disease clinical trials: results of a 3‐month comparative study using the Breathlessness, Cough and Sputum Scale (BCSS). Respiratory Medicine 2003;97:S35‐S43. - PubMed
Chapman 2002 {published and unpublished data}
-
- Chapman K, Arvidsson P, Chuchalin A, Dhillon D, Faurschou P, Goldstein R, et al. The addition of salmeterol 50 mcg bid to anticholinergic treatment in patients with COPD: a randomised placebo controlled trial. Canadian Respiratory Journal 2002;9(3):178‐85. - PubMed
-
- Chapman K, James MH, Kuipers AF, Goldstein R. Addition of salmeterol 50 mcg bid to anticholinergic treatment in COPD. American Journal of Respiratory & Critical Care Medicine 1999;159(3):A523.
-
- SLGF53. A multi‐centre, double‐blind, parallel group study to evaluate the efficacy of serevent 50mg bid versus placebo bid all administered via the multi dose powder inhaler (diskus/accuhaler) in terms of symptoms in the treatment of patients with chronic obstructive pulmonary disease. GlaxoSmithKline Clinical Trials Register (http:ctr.gsk.co.uk) 2005.
Dal Negro 2003 {published and unpublished data}
-
- Dal Negro R, Pomari C, Tognella S, Micheletto C. Salmeterol & fluticasone 50 microg/250 microg bid in combination provides a better long‐term control than salmeterol 50 microg bid alone and placebo in COPD patients already treated with theophylline. Pulmonary Pharmacology & Therapeutics 2003;16:241‐6. - PubMed
Dauletbaev 2001 {published data only}
-
- Dauletbaev N, Viel K, Bargon J. Salmeterol or ipratropium bromide/fenoterol in stable mild‐moderate COPD. European Respiratory Journal 2001;18(Suppl 33):426s.
-
- SMS40308. A randomised, double‐blind, double‐dummy, placebo‐controlled, parallel‐group, multicenter study to compare efficacy and tolerability of salmeterol (50 µg b.i.d., Serevent® Diskus®) and a combination of ipratropium/fenoterol (40/100 µg q.i.d., Berodual® MDI) in patients with mild‐to‐moderate chronic obstructive lung disease (COPD). GlaxoSmithKline Clinical Trials Register (http:ctr.gsk.co.uk) 2005.
Grove 1996 {published and unpublished data}
Gupta 2002 {published data only}
-
- Gupta R, Chhabra S. An evaluation of salmeterol in the treatment of chronic obstructive pulmonary disease. The Indian Journal of Chest Diseases & Allied Sciences 2002;44:165‐72. - PubMed
Hanania 2003 {published data only}
-
- Hanania N, Darken P, Horstman D, Reisner C, Lee B, Davis S, et al. The efficacy and safety of fluticasone propionate (250 ug)/salmeterol (50 ug) combined in the Diskus Inhaler for the treatment of COPD. Chest 2003;124:834‐43. - PubMed
-
- Hanania NA, Ramsdell J, Payne K, Davis S, Horstman D, Lee B, et al. Improvements in airflow and dyspnea in COPD patients following 24 weeks treatment with salmeterol 50mcg and fluticasone propionate 250mcg alone or in combination via the diskus. American Journal of Respiratory & Critical Care Medicine 2001;163(5 Suppl):A279.
-
- Horstman D, Darken P, Davis S, Lee B. Improvements in FEV1 and symptoms in poorly‐reversible COPD patients following treatment with salmeterol 50mcg/fluticasone propionate 250mcg combination. European Respiratory Journal 2003;22(Suppl 45):50s.
-
- Mahler DA, Darken P, Brown CP, Knobil K. Predicting lung function responses to combination therapy in chronic obstructive pulmonary disease (COPD) [Abstract]. National COPD Conference; Arlington, Virginia. 2003:Abstract no: 1081.
-
- Mahler DA, Darken P, Brown CP, Knobil K. Predicting lung function responses to salmeterol/fluticasone propionate combination therapy in COPD [Abstract]. European Respiratory Journal 2003;22(Suppl 45):P429.
Mahler 1999 {published and unpublished data}
-
- Goodwin B, Cox F, Anderson W, Wisniewski M, Rickard K. Comparison of salmeterol 42ug BID (SLG) versus ipratropium bromide 36 ug q.i.d. vs placebo on disease specific quality of life in COPD patients: reversible or non‐reversible to ventolin. 1997‐1999 ALA/ATS Abstracts‐on‐disk.. 1997.
-
- Mahler DA, Donohue JF, Barbee RA, Goldman M, Gross NJ, Wisniewski ME, et al. Efficacy of salmeterol xinafoate in the treatment of COPD. Chest 1999;115:957‐65. - PubMed
-
- SLGA4005. A randomized, double‐blind, double‐dummy, comparative clinical trial of 12‐week courses of salmeterol xinafoate versus ipratropium bromide versus placebo (prn ventolin®) in subjects with chronic obstructive pulmonary disease. GlaxoSmithKline Clinical Trials Register (http:ctr.gsk.co.uk) 2005.
Mahler 2002 {published data only}
-
- Mahler DA, Darken P, Brown CP, Knobil K. Predicting Lung Function Responses to Combination Therapy in Chronic Obstructive Pulmonary Disease (COPD) Predicting Lung Function Responses to Combination Therapy in Chronic Obstructive Pulmonary Disease (COPD). http://www.abstracts2view.com. 2003.
-
- Mahler DA, Wire P, Horstman D, Chang CN, Yates J, Fischer T, Shah T. Effectiveness of fluticasone propionate and salmeterol combination delivered via the Diskus device in the treatment of chronic obstructive pulmonary disease. American Journal of Respiratory & Critical Care Medicine 2002;166:1084‐91. - PubMed
-
- SFCA3006. A randomized, double‐blind, placebo‐controlled, parallel‐group trial evaluating the safety and efficacy of the diskus formulations of salmeterol (sal) 50mcg bid and fluticasone propionate (fp) 500mcg bid individually and in combination as salmeterol 50mcg/fluticasone propionate 500mcg bid (sfc 50/500) compared to placebo in COPD subjects. GlaxoSmithKline Clinical Trials Register (http:ctr.gsk.co.uk) 2005.
-
- Spencer M, Wire P, Lee B, Chang CN, Darken P, Horstman D. Patients with COPD using salmeterol/fluticasone propionate combination therapy experience improved quality of life. European Respiratory Journal 2003;22(Suppl 45):51s.
-
- Spencer MD, Anderson JA. Salmeterol/fluticasone combination produces clinically important benefits in dyspnea and fatigue [Abstract]. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005:B93, Poster: 308.
Rennard 2001 {published and unpublished data}
-
- Goodwin B, Cox F, Anderson W, Wisniewski M, Rickard K. Evaluation of quality of life in reversible and non‐reversible patients with COPD receiving salmeterol xinafoate 42mcg BID (SLG) versus ipratropium bromide 36mcg QID (IP) versus placebo (PL). American Journal of Respiratory & Critical Care Medicine. 1997; Vol. 155:A724.
-
- Rennard S, Anderson W, ZuWallack R, Broughton J, Bailey W, Friedman M, et al. Use of a long‐acting inhaled Beta2‐adrenergic agonist, salmeterol xinafoate, in patients with chronic obstructive pulmonary disease. American Journal of Respiratory & Critcal Care Medicine 2001;163(5):1087‐92. - PubMed
-
- SLGA4004. A randomized, double‐blind, double‐dummy, comparative clinical trial of 12‐week courses of salmeterol xinafoate versus ipratropium bromide versus placebo (prn ventolin) in subjects with chronic obstructive pulmonary disease. GlaxoSmithKline Clinical Trials Register (http:ctr.gsk.co.uk) 2005.
Rossi 2002 {published and unpublished data}
-
- Rossi A, Thomson M, Della Cioppa G. Comparison of the efficacy, tolerability and safety of formoterol dry powder and oral, slow‐release theophylline in the treatment of COPD. Chest 2002;121:1058‐69. - PubMed
SCO40030 {unpublished data only}
-
- SCO40030. A randomized, double‐blind, placebo‐controlled, parallel group clinical trial evaluating the effect of the fluticasone propionate/salmeterol combination product 250/50mcg bid via diskus and salmeterol 50mcg bid via diskus on lung hyperinflation in subjects with chronic obstructive pulmonary disease (COPD). GlaxoSmithKline Clinical Trials Register (http:ctr.gsk.co.uk) 2005.
SMS40298 {published data only}
-
- SMS40298. A multi‐centre, randomized, double‐blind, parallel group study to evaluate the impact on Quality of Life (QOL) of adding Serevent 50ug bid via MDI to patients’ existing therapy in patients with chronic obstructive pulmonary disease (COPD). GlaxoSmithKline Clinical Trials Register (http:ctr.gsk.co.uk) 2005.
SMS40314 {published data only}
-
- SMS40314. A multicenter, randomized, double‐blind, double‐dummy, parallel‐group, 8‐week comparison of salmeterol xinafoate versus ipratropium bromide versus salmeterol xinafoate plus ipratropium bromide versus placebo in subjects with chronic obstructive pulmonary disease. GlaxoSmithKline Clinical Trials Register (http:ctr.gsk.co.uk) 2005.
SMS40315 {published data only}
-
- SMS40315. A multicenter, randomized, double‐blind, double‐dummy, parallel group, 8‐week comparison of salmeterol xinafoate versus ipratropium bromide versus salmeterol xinafoate plus ipratropium bromide versus placebo in subjects with chronic obstructive pulmonary disease. GlaxoSmithKline Clinical Trials Register (http:ctr.gsk.co.uk) 2005.
SMS40318 {published data only}
-
- SMS40318. Randomized, double blind, placebo‐controlled, parallel‐group trial over 4 weeks to evaluate the effect of salmeterol (2x50 µg/d by Diskus®) on the lung volumes at rest and during sub‐maximal exercise in subjects with moderate chronic obstructive pulmonary disease (COPD). GlaxoSmithKline Clinical Trials Register (http:ctr.gsk.co.uk) 2005.
Stockley 2002 {unpublished data only}
-
- SMS40026. A multi‐centre, randomised, double‐blind, placebo‐controlled, parallel‐group study to investigate the effect of 12 months treatment with salmeterol (50mcg bd), delivered via the diskus* inhaler, on the incidence of moderate and severe exacerbations in subjects with chronic obstructive pulmonary disease (COPD) when added to their usual treatment regimen. GlaxoSmithKline Clinical Trials Register (http:ctr.gsk.co.uk) 2005.
-
- Stockley R, Chopra N. Salmeterol added to usual therapy, is an effective bronchodilator over 12 months of treatment in chronic obstructive pulmonary disease [abstract]. European Respiratory Society Annual Congress. 2002:P1568.
-
- Stockley R, Rice L, Chopra N. Salmeterol added to usual therapy gives a rapid improvement in lung function that is sustained at 6 and 12 months in patients with COPD [abstract]. European Respiratory Society Annual Congress. 2002:P1567.
-
- Stockley R, Rice L, Chopra N. Salmeterol added to usual therapy significantly reduces the frequency of moderate/severe exacerbations in patients with COPD [abstract]. American Thoracic Society 99th International Conference. 2003:A108, Poster C51.
Ulrik 1995 {published data only}
-
- SLGL17. A single centre, randomised, double‐blind, cross‐over study to compare the efficacy of inhaled salmeterol xinafoate dry powder 50mg bid via the diskhaler with placebo dry powder bid via the diskhaler in the treatment of non‐reversible COPD patients and to provoke with histamine and methacholine before and after treatment to investigate whether salmeterol can reduce the bronchial hyperreactivity. GlaxoSmithKline Clinical Trials Register (http:ctr.gsk.co.uk) 2005.
van Noord 2000 {published data only}
-
- Noord JA, Munck DR, Bantje TA, Hop WCJ, Bommer AM. Long‐term treatment of chronic obstructive pulmonary disease with salmeterol and the additive effect of ipratropium. European Respiratory Journal 2000;15(5):878‐85. - PubMed
Wadbo 2002 {published and unpublished data}
-
- Stahl E, Wadbo M, Bengtsson T, Strom K, Lofdahl C‐G. Health‐related quality of life, symptoms, exercise capacity and lung function during treatment for moderate to severe COPD. Journal of drug assessment 2002;5:81‐94.
-
- Stahl E, Wadbo M, Bengtsson T, Strom K, Lofdahl CG. Health‐related quality of life, symptoms, exercise capacity and lung function during treatment for moderate to severe COPD. Journal of Outcomes Research 2001;5:11‐24.
-
- Wadbo M, Lofdahl CG, Larsson K, Skoogh BE, Tornling G, Arwestrom E, et al. Effects of formoterol and ipratropium bromide in COPD: a 3‐month placebo‐controlled study. European Respiratory Journal 2002;20:1138‐46. - PubMed
References to studies excluded from this review
Aalbers 2002 {published data only}
-
- Aalbers R, Ayres J, Backer V, Decramer M, Lier PA, Magyar P, et al. Formoterol in patients with chronic obstructive pulmonary disease: a randomized, controlled, 3‐month trial. European Respiratory Journal 2002;19:936‐43. - PubMed
Anderson 1997 {published data only}
-
- Anderson WH, Wisniewski M, Anzueto A, Jenkinson S, Goldman M, Ramsdell J, et al. The safety and efficacy of salmeterol in patients with COPD: Results from SLGA4005, a multicentre trial. Chest 1997;112(3 Suppl):79s‐80s.
Bailey 1997 {published data only}
-
- Bailey W, Yancey S, Rickard K. Peak flow and symptom monitoring in COPD: a 12 week comparison of placebo, Atrovent and Serevent. American Journal or Respiratory & Critical Care Medicine 1997;155(4):A278.
Barnes 1993 {published data only}
-
- Barnes NC. New developments in the treatment of asthma and chronic obstructive pulmonary disease. Respiratory Medicine 1993;87(Suppl B):53‐6. - PubMed
Boulet 1992 {published data only}
-
- Boulet LP. Bronchodilator effect of salmeterol and inhibition of bronchial hyperreactivity. Revue des Maladies Respiratoires 1992;9(Suppl 1):R11‐13. - PubMed
Brogden 1992 {published data only}
-
- Brogden RN, Faulds D. Salmeterol xinafoate: Aareview of its pharmacological properties and therapeutic potential in reversible obstructive airways disease. Drugs 1991;42(5):895‐912. - PubMed
Brusasco 2003 {published data only}
Calverley 2003a {published and unpublished data}
-
- Borgstrom L, Asking L, Olsson H, Peterson S. Lack of interaction between disease severity and therapeutic response with budesonide/formoterol in a single inhaler [Abstract]. American Thoracic Society 100th International Conference, May 21‐26. 2004:C22, Poster 505.
-
- Calverley PMA, Olsson H, Symbicort International COPD Study Group. Budesonide/formoterol ina single inhaler sustains improvements in lung function over 12 months compared with monocomponents and placebo in patients with COPD [abstract]. American Thoracic Society 99th International Conference. 2003:B024, Poster 418.
-
- Calverley PMA, Peterson S. Combining budesonide/formoterol in a single inhale reduces exacerbation frequency in COPD [abstract]. American Thoracic Society 99th International Conference. 2003:D092, Poster 211.
-
- Calverley PMA, Thompson NC, Olsson H. Budesonide/formoterol in a single inhaler sustains lung function improvements in COPD [Abstract]. European Respiratory Journal 2003;22(Suppl 45):P435.
-
- Calverly PM, Bonsawat W, Cseke Z, Zhong N, Peterson S, Olsson H. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. European Respiratory Journal 2003;22:912‐9. - PubMed
Cazzola 1994 {published data only}
-
- Cazzola M, Santangelo G, Piccolo A, Salzillo A, Matera MG, D'Amato G, et al. Effects of salmeterol and formoterol in patients with chronic obstructive pulmonary disease. Pulmonary Pharmacology 1994;7:103‐7. - PubMed
Cazzola 1995 {published data only}
-
- Cazzola M, Matera MG, Santangelo G, Vinciguerra A, Rossi F, D'Amato G. Salmeterol and formoterol in partially reversible severe chronic obstructive pulmonary disease: a dose‐response study. Respiratory Medicine 1995;89:357‐62. - PubMed
Chazan 1995 {published data only}
-
- Chazan R, Jaworski A, Grubek‐Jaworska H, Droszcz W. Effect of 16 week use of salmeterol on ECP levels, pulmonary function tests and bronchial hyperreactivity in patients with chronic obstructive pulmonary disease. Polski Tygodnik Lekarski 1995;50(40‐44):48‐49. - PubMed
D'Urzo 2001 {published data only}
-
- D'Urzo AD, Salvo MC, Ramirez‐Rivera A, Almeida J, Sichletidis L, Rapatz G, Kottakis J, FOR‐INT‐03 Study Group. In patients with COPD, treatment with a combination of formoterol and ipratropium is more effective than a combination of salbutamol and ipratropium : a 3‐week, randomized, double‐blind, within‐patient, multicenter study. Chest 2001;119(5):1347‐56. - PubMed
Dahl 2001 {published data only}
-
- Dahl R, Greefhorst L, Nowak D, Nonikov V, Byrne A, Thomson M, et al. Inhaled formoterol dry powder versus ipratropium bromide in chronic obstructive pulmonary disease. American Journal of Respiratory & Critical Care Medicine 2001;164:778‐84. - PubMed
Del Torre 1992 {published data only}
-
- Torre L, Melica EV, Torre M. Effectiveness of salmeterol in patients with emphysema. Current Therapeutic Research 1992;52(6):888‐98.
Donohue 2002 {published data only}
-
- Donohue J, Bateman E, Lee A, Kesten S. A 6‐month, placebo‐controlled study comparing lung function and health status changes in copd patients treated with tiotropium or salmeterol. Chest 2002;122:47‐55. - PubMed
Germouty 1992 {published data only}
-
- Germouty J, Aubert J, Clavier J, Paramelle B, Voisin C. Tolérance à longe terme du Formotérol chez des bronchopathes chroniques obstructifs. Allergie et Immunologie 1992;24(9):342‐7. - PubMed
Howder 1993 {published data only}
-
- Howder CL. Anti‐muscarinic and Beta2 adrenoceptor bronchodilators in obstructive airways disease. Respiratory Care 1993;38(12):1364‐88.
Joseph 1994 {published data only}
-
- Joseph JC. Chronic obstructive pulmonary disease U.S. Pharmacist 1994;19(12):H3‐14.
Kotaniemi 1994 {published data only}
-
- Kotaniemi JT. Salmeterol in asthma and COPD: a long term follow‐up. American Journal of Critical Care Medicine 1994;149(4):A207.
Mahler 1997 {published data only}
-
- Mahler D, ZuWallach R, Rickard K, Yancey S, Wisniewski M, Anderson W. Effects of salmeterol and ipratropium bromide on dyspnea as measured by the six minute walk and baseline dyspnea index/transitional dyspnea index (BDI/TDI). American Journal of Respiratory & Critical Care Medicine 1997;155:A278.
Maltais 1995 {published data only}
-
- Maltais F, Bourbeau J. Medical management of emphysema. Chest Surgery Clinics of North America 1995;5(4):673‐89. - PubMed
Matera 1995 {published data only}
-
- Matera MG, Cazzola M, Vinciguerraaa A, Perna F, Calderaro F, Caputi M, et al. Comparison of hte bronchodilating effects of salmeterol, salbutamol and ipratropium bromide in patients with chronic obstructive pulmonary disease. Pulmonary Pharmacology 1995;8:267‐71. - PubMed
Matera 1996 {published data only}
-
- Matera MG, Caputi M, Cazzola M. A combination with clinical recommended dosages of salmeterol and ipratropium is not more effective than salmeterol alone in patients with chronic obstructive pulmonary disease. Respiratory Medicine 1996;90:497‐9. - PubMed
Melani 1996 {published data only}
-
- Melani AS, Pirelli M, Gregorio A. Effects of inhaled salmeterol and orally dose‐titrated theophylline on exercise capaity of stable COPD patients. European Respiratory Journal 1996;9:391s.
Newman 1996 {published data only}
-
- Newman AM, Wiggins J, Smith M, Taak NK, Barnes P. Salmeterol xinafoate (100 mcg BD) in the treatment of chronic obstructive pulmonary disease (COPD). European Respiratory Journal 1996;9:183s.
Patakas 1995 {published data only}
-
- Patakas D, Andreadis D, Mavrofridis E, Argyropoulou P, Sarigiannidis A. Comparison of the effect of salmeterol and ipratropium bromide, on exercise performance and breathlessness in patients with chronic obstructive pulmonary disease (COPD). European Respiratory Journal 1995;8(Suppl 19):94s. - PubMed
Pingleton 1996 {published data only}
-
- Pingleton S. Pulmonary Medicine. JAMA 1996;275(23):1849‐50. - PubMed
Ramirez‐Venegas 1997 {published data only}
-
- Ramirez‐Venegas A, Ward J, Lentine T, Mahler D. Salmeterol reduces dyspnea and improves lung function in patients with COPD. Chest 1997;112:336‐40. - PubMed
Schultze‐Wern.1990 {published data only}
-
- Schultze‐Werninghaus G. Multicentre 1‐year trial on Formoterol, a new long acting Beta2‐agonist, in chronic obstructive airways disease. Lung 1990;168(Suppl):83‐9. - PubMed
Schultze‐Wern. 1996 {published data only}
-
- Schultze‐Werninghaus G. Salmeterol, a long‐acting beta2‐agonist in airways obstruction. Effects, side effects and therapeutic assessment [Der langwirksame inhalative Beta2‐agonist Salmeterol bei obstrucktiven Atemwegserkrankungen. Wirkungen, Nebenwirkungen und Stellenwert]. Allegro Journal 1996;5(8):453‐60.
SLMF 4010 {published data only}
-
- SLMF 4010. Multicentre, randomised, parallel group, placebo‐controlled, double‐blind, study, stratified on tobacco status at enrollment, evaluating during 6 months the efficacy of salmeterol powder for inhalation, 50 µg two times per day for the reduction of thoracic distension in subjects with chronic obstructive pulmonary disease (COPD). GlaxoSmithKline Clinical Trials Register (http:ctr.gsk.co.uk) 2005.
Szafranski 2003 {published data only}
-
- Szafranski W, Cukier A, Ramirez A, Menga G, Sansores R, Nahabedian S, et al. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. European Respiratory Journal 2003;21:74‐81. - PubMed
Vollmer 1995a {published data only}
-
- Vollmer M, Schmidt EW, Ulmer WT. Effect duration and treatment effectiveness of salmeterol, fenoterol and salbutamol in severe forms of respiratory tract obstruction. Pneumologie 1995;49(10):528‐34. - PubMed
Vollmer 1995b {published data only}
-
- Vollmer M, Schmidt EW, Ulmer WT. Responder and non‐responder in the bronchodilator test? Variability of short‐term responses to beta2‐adrenoceptor agonists in chronic airways obstruction. Pneumologie 1995;49(11):584‐9. - PubMed
References to studies awaiting assessment
Della Cioppa 2001 {unpublished data only}
-
- Della Cioppa G, Byrne A, Till D, Wood R. Formoterol demonstrates bronchodilator efficacy in patients with reversible or poorly reversible chronic obstructive pulmonary disease, regardless of concomitant corticosteroid use [abstract]. European Respiratory Journal 2001;18(Suppl 33):177s.
Greefhorst 2000 {published data only}
-
- Greefhorst AP, Dahi R, Nowak D, Nonikov V, Byrne A, Colcchio C, et al. Effect of inhaled formoterol and ipratropium bromide on quality of life, "bad days" and exacerbations in patients with COPD. American Thoracic Society 2000 Toronto (Abstracts‐On‐Disk). 2000.
Kristufek 2001 {unpublished data only}
-
- Kristufek P, Levine B, Till D, Byrne A. Inhaled formoterol (Foradil(r)) improves lung function in patients with both reversible and poorly reversible COPD [abstract]. American Journal of Respiratory and Critical Care Medicine 2001;163(5 Suppl):A280.
Littner 2001 {unpublished data only}
-
- Littner M, Yates J, Fischer T, Horstman D, Wire P. Improvements in FEV1 and symptoms in poorly‐reversible COPD patients following 24 weeks of treatment with the salmeterol/fluticasone propionate combination 50/500 BID via the Diskus Inhaler [abstract]. European Respiratory Journal 2001;18(Suppl 33):176s.
Sybrecht 1999 {published data only}
-
- Sybrecht GW. Inhaled formoterol was an effective and safe treatment in COPD patients. European Respiratory Society Annual Congress (Abstracts‐on‐Disk). 1999:P2510.
References to ongoing studies
TORCH {published data only}
-
- The TORCH Study Group. The TORCH (TOwards a Revolution in COPD Health) survival study protocol. European Respiratory Journal 2004;24:206‐10. - PubMed
Additional references
ATS/ERS 2004
-
- Celli BR, MacNee W, ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. European Respiratory Journal 2004;23:932‐46. - PubMed
Barr 2005
Calverley 2003b
Cates 2002
GOLD 2001
-
- Pauwels RA, Buist AS, Calverley PM, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. American Journal of Respiratory Critical Care Medicine 2001;163:1256–76. - PubMed
Guyatt 1988
-
- Guyatt G, Townsend M, Nogradi S, Pugsley S, Keller J, Newhouse M. Acute response to bronchodilator. An imperfect guide for bronchodilator therapy in chronic airflow limitation. Archives of Internal Medicine 1988;148:1949‐52. - PubMed
Handbook 2008
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org. The Cochrane Collaboration, 2008.
Higgins 2003
Jadad 1996
-
- Jadad AR, Moore A, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomised controlled trials: Is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. - PubMed
Jones 1991
-
- Jones PW, Quirk FH, Baveystock CM. The St George's Respiratory Questionnaire. Respiratory Medicine 1991;85:25‐31. - PubMed
Jones 1992
-
- Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self‐complete measure of health status for chronic airflow limitation. American Review of Respiratory Diseases 1992;145:1321‐7. - PubMed
Karner 2012
Nannini 2004
-
- Nannini L, Cates CJ, Lasserson TJ, Poole P. Combined corticosteroid and long‐acting beta‐agonist in one inhaler for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2004, Issue 3. - PubMed
Ni Chroinin 2005
-
- Ni Chroinin M, Greenstone IR, Danish A, Magdolinos H, Masse V, Zhang X, Ducharme FM. Long‐acting beta2‐agonists versus placebo in addition to inhaled corticosteroids in children and adults with chronic asthma. The Cochrane Database of Systematic Reviews 2005, Issue 4. - PubMed
NICE/BTS 2004
O'Donnell 1997
-
- O'Donnell D, Bertley J, Chau L, Webb K. Qualitative aspects of exertional breathlessness in chronic airflow limitation: pathophysiological mechanisms. American Journal of Respiratory & Critical Care Medicine 1997;155:109‐15. - PubMed
O'Donnell 1999
-
- O'Donnell D, Lam M, Webb K. Spirometric correlates of improvement in exercise performance after anticholinergic therapy in chronic obstructive pulmonary disease. American Journal of Respiratory & Critical Care Medicine 1999;160:542‐9. - PubMed
Reidelmeier 1996
-
- Redelmeier DA, Guyatt GH, Goldstein RS. Assessing the minimal important difference in symptoms: a comparison of two techniques. Journal of Clinical Epidemiology 1996;49(11):1215‐19. - PubMed
Spencer 2004
-
- Spencer S, Calverley PM, Burge PS, Jones PW. Impact of preventing exacerbations on deterioration of health status in COPD. European Respiratory Journal 2004;23(5):698‐702. - PubMed
Sterne 2001
Tashkin 2003
-
- Tashkin D, Kesten S. Long‐term treatment benefits with tiotropium in COPD patients with and without short‐term bronchodilator responses. Chest 2003;123:1441‐9. - PubMed
Verberne 1998
-
- Verberne AA, Fuller R. An overview of nine clinical trials of salmeterol in an asthmatic population. Respiratory Medicine 1998;92:777‐82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

