Polyunsaturated fatty acid supplementation for schizophrenia
- PMID: 16855961
- PMCID: PMC7032618
- DOI: 10.1002/14651858.CD001257.pub2
Polyunsaturated fatty acid supplementation for schizophrenia
Abstract
Background: Limited evidence supports a hypothesis suggesting that schizophrenic symptoms may be the result of altered neuronal membrane structure and metabolism. The structure and metabolism is dependent on blood plasma levels of certain essential fatty acids and their metabolites.
Objectives: To review the effects of polyunsaturated fatty acids for people with schizophrenia.
Search strategy: We have updated the initial searches of 1998 and 2002 (Cochrane Schizophrenia Group's Register, July 2005), and where necessary, we contacted authors and relevant pharmaceutical companies.
Selection criteria: We included all randomised clinical trials of polyunsaturated fatty acid treatment for schizophrenia.
Data collection and analysis: Working independently, we selected studies for quality assessment and extracted relevant data. We analysed on an intention-to-treat basis. Where possible and appropriate we calculated the Relative Risk (RR) and their 95% confidence intervals (CI) and estimated the number needed to treat (NNT). For continuous data we calculated weighted mean differences (WMD) and their 95% confidence intervals. We also inspected the data for heterogeneity.
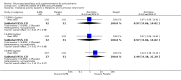
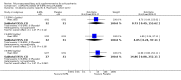
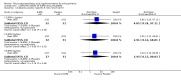
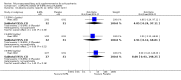
Main results: When any dose omega-3 (E-EPA or EPA) is compared with placebo, small short trials suggest that the need for neuroleptics appears to be reduced for people allocated omega-3 supplementation (n=30, 1 RCT, RR 0.73 CI 0.54 to 1.00) and mental state may improve (n=30, 1 RCT, RR not gaining 25% change in PANSS scores 0.54 CI 0.30 to 0.96, NNT3 CI 2-29). There are no differences in the number of people leaving the study early (n=271, 4 RCTs, RR 0.91 CI 0.36 to 2.33). There are few data on the comparison of any dose omega-6 (GLA) with placebo. For movement disorder outcomes, the only small study we found does not show any difference for average short-term endpoint AIMS score (n=16, 1 RCT, MD 1.30 CI -1.96 to 4.56). When any dose omega 3 (E-EPA or EPA) is compared with any dose omega-3 (DHA) there is no clear difference for mental state outcome of not gaining 25% change in PANSS scores (n=31, 1 RCT, RR 0.66 CI 0.39 to 1.11). When different doses of omega-3 (E-EPA) are compared with placebo there are no differences in measures of global and mental state between the studies. For the outcome of 'experiencing at least one adverse effect' no differences between groups are found for any dose (1g/day E-EPA vs placebo n=63 1 RCT, RR 0.97 CI 0.60 to 1.56; 2g/day E-EPA vs placebo n=63 1 RCT, RR 0.67 CI 0.37 to 1.20; 4g/day E-EPA vs placebo n=58, 1 RCT, RR 1.15 CI 0.72 to 1.82).
Authors' conclusions: Two updates of this review have resulted in more included studies but relatively little useful additional data. The results remain inconclusive. The new trials all compare the omega-3 polyunsaturated fatty acids, in particular eicosapentaenoic acid and its ester, ethyl-eicosapentaenoic acid. The use of omega-3 polyunsaturated fatty acids for schizophrenia still remains experimental and this review highlights the need for large well designed, conducted and reported studies.
Conflict of interest statement
None known.
Figures
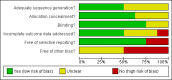
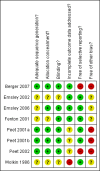





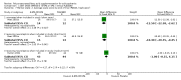











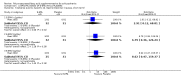







Update of
-
Polyunsaturated fatty acid supplementation for schizophrenia.Cochrane Database Syst Rev. 2003;(2):CD001257. doi: 10.1002/14651858.CD001257. Cochrane Database Syst Rev. 2003. Update in: Cochrane Database Syst Rev. 2006 Jul 19;(3):CD001257. doi: 10.1002/14651858.CD001257.pub2. PMID: 12804400 Updated.
References
References to studies included in this review
Berger 2007 {published data only}
-
- Berger G, Wood S, Proffitt T, McConchie M, Khan A, O'Donnell C, Yuen H, Smith D, Horrobin D, McGorry P. Ethyl‐eicosapentaenoic acid (E‐EPA) supplementation in early psychosis. A double‐blind add on standard therapy in 80 drug naive or early treated first episode psychosis patients. Schizophrenia Research 2004;70(1):41.
-
- Berger GE, Proffitt TM, McConchie M, Yuen H, Wood SJ, Amminger GP, Brewer W, McGorry PD. Ethyl‐eicosapentaenoic acid in first‐episode psychosis: a randomised placebo‐controlled trial. Journal of Clinical Psychiatry 2007;68(12):1867‐75. - PubMed
-
- Berger GE, Proffitt TM, McConchie MA, Wood SJ, Yuen HP, McGorry PD. Ethyl‐Eicosapentaenoic acid (E‐EPA) supplementation in early psychosis. A double‐blind, randomised, placebo controlled trial (RCT) comparing 2g E‐EPA versus placebo add on therapy in 80 drug naive or early treated first episode psychosis. Schizophrenia Bulletin 2005;31:475.
-
- McConchie MA, Berger GE, Proffitt TM, Yuen HP, Wood S, smith D, Horrobin D, McGorry PD. Effect of diagnostic heterogeneity on response to ethyl‐eicosapentaenoic acid (EPA) in first episode psychosis (FEP). Proceedings of the 12th Biennial Winter Workshop on Schizophrenia; 2004 Feb 7‐13, Davos, Switzerland. Davos, 2004.
-
- Proffitt T, Wood S, Yuen H, McConchie M, Brewer W, Horrobin D, McGorry P, Berger G. Ethyl‐Eicosapentaenoic acid (E‐EPA) supplementation in first‐episode psychosis: effects on cognition mediated by lipid metabolic status. Schizophrenia Research 2004;70(1):40.
Emsley 2002 {published data only}
-
- Emsley R, Myburgh C, Oosthuizen P, Rensburg SJ. Randomised, placebo‐controlled study of ethyl‐eicosapentaenoic acid as supplemental treatment in schizophrenia. American Journal of Psychiatry 2002;159:1596‐8. - PubMed
Emsley 2006 {published data only}
-
- Emsley R. A double‐blind, randomised, parallel‐group comparison of ethyl‐eicosapentaenoic acid (e‐epa) versus placebo as add‐on medication in patients with established tardive dyskinesia. www.clinicaltrials.gov 2005.
-
- Emsley R, Niehaus DJH, Koen L, Oosthuizen PP, Turner HJ, Carey P, Rensburg SJ, Maritz JS, Murck H. The effects of eicosapentaenoic acid in tardive dyskinesia: a randomised placebo‐controlled trial. Schizophrenia Research 2006;84(1):112‐20. - PubMed
Fenton 2001 {published data only}
-
- Fenton W, Dickerson F, Boronow JJ, Hibbeln JR, Knable MB. A placebo‐controlled trial of omega‐3 fatty acid (ethyl eicosapentaenoic acid) supplementation for residual symptoms and cognitive impairment in schizophrenia. American Journal of Psychiatry 2001;158(12):2071‐4. - PubMed
-
- Fenton WS, Boronow J, Dickerson F, Hibbein J, Knable MB. Randomised trial of supplemental EPA for residual symptoms of schizophrenia. Biological Psychiatry 2000;47:S159‐60.
-
- Fenton WS, Dickerson F, Boronow JJ, Hibbein JR, Knable MB. Placebo‐controlled trial of omega‐3 fatty acid. Proceedings of the 155th Annual Meeting of the American Psychiatric Association; 2002 May 18‐23; Philadelphia,USA. Philadelphia, 2002.
Peet 2001a {published data only}
-
- Peet M. International multi‐centre, double‐blind, placebo controlled trial of EPA as an adjunct to existing neuroleptic treated schizophrenic patients. National Research Register 2001.
-
- Peet M, Brind J, Ramchand CN, Shah S, Vankar GK. Two double‐blind placebo‐controlled pilot studies of eicosapentaenoic acid in the treatment of schizophrenia. Schizophrenia Research 2001;49:243‐51. - PubMed
-
- Peet M, Laugharne J, Mellor J. Double‐blind trial of N3 fatty acid supplementation in the treatment of schizophrenia. Schizophrenia Research 1997;24:209.
-
- Peet M, Mellor J. Double‐blind, placebo controlled trial of N‐3 polyunsaturated fatty acids as an adjunct to neuroleptics. Schizophrenia Research 1998;29:160.
-
- Shah S, Vankar GK, Telang SD, Ramchand CN, Peet M. Eicosapentaenoic acid (EPA) as an adjunct in the treatment of schizophrenia. Schizophrenia Research 1998;29:158. - PubMed
Peet 2001b {published and unpublished data}
-
- Peet M, Brind J, Ramchand CN, Sha S, Vankar GK. Two double‐blind placebo‐controlled pilot studies of eicosapentaenoic acid in the treatment of schizophrenia. Schizophrenia Research 2001;49:243‐51. - PubMed
Peet 2002 {published and unpublished data}
-
- Horrobin DF. The role of phospholipids in schizophrenia. Proceedings of the 7th World Congress of Biological Psychiatry; 2001 July 1‐6; Berlin, Germany. Berlin, 2001.
-
- Horrobin DF, Bennett CN, Peet M. Correlation between clinical improvement and red cell fatty acid changes when treating schizophrenia with eicosapentaenoic acid. Proceedings of the 8th International Congress on Schizophrenia Research; 2001 April 28‐ May 2; British Columbia, Canada. British Columbia, 2001:232.
-
- Horrobin Df, Jenkins K, Bennett CN, Christie WW. Eicosapentaenoic acid and arachidonic acid: collaboration and not antagonism is the key to biological understanding. Prostaglandins, Leukotrienes and Essential Fatty Acids 2002;66(1):83‐90. - PubMed
-
- Peet M. A multi‐centre, double‐blind, randomised, parallel group, placebo controlled, dose ranging pilot study of ethyl eicosapentaenoate (ethly EPA) in patients with schizophrenia. National Research Register 2001.
-
- Peet M. Eicosapentaenoic acid (EPA) is effective in relieving schizophrenic symptoms in patients on clozapine. Proceedings of the 8th International Congress on Schizophrenia Research; 2001 April 28 ‐ May 2; British Columbia, Canada. British Columbia, 2001:241‐2.
Wolkin 1986 {published data only}
-
- Wolkin A, Jordan B, Peselow E, Rubinstein M, Rotrosen J. Essential fatty acid supplementation in tardive dyskinesia. American Journal of Psychiatry 1986;143:912‐4. - PubMed
References to studies excluded from this review
Amminger 2007 {published data only}
-
- Amminger GP. Indicated prevention with omega‐3 fatty acids in adolescents with 'at‐risk‐mental‐state' for psychosis: a randomised, double blind, placebo‐controlled treatment trial. www.clinicaltrials.gov 2006.
-
- Amminger GP Schafer MR. Is it feasible to conduct a RCT in ultra‐high risk individuals at a child and adolescent psychiatric service?. Proceedings of the 5th International Conference on Early Psychosis; 2006 Oct 4‐6; Birmingham, UK. Birmingham, 2006.
-
- Amminger GP, Schafer MR. Indicated prevention with omega‐3 fatty acids in adolescents at ultra‐high risk for psychosis ‐ rationale, methods and 3‐months outcome. Proceedings of the 5th International Conference on Early Psychosis; 2006 Oct 4‐6, Birmingham, UK. Birmingham, 2006.
-
- AmmingerG, Schaefer MR, Papageorgiou K, Becker J, Mossaheb N, Harrigan SM, McGorry PD, Berger GE. Omega‐3 fatty acids reduce the risk of early transition to psychosis in ultra‐high risk individuals: a double‐blind randomised, placebo‐controlled treatment study. Schizophrenia Bulletin 2007;33(2):418‐9.
-
- Klier C, Hollmann M, Schlogelhofer M, Mossaheb N, Friedrich M, Amminger PG. Indicated prevention with omega‐3 fatty acids (epa/dha) in adolescents with "at‐risk‐mental‐state" for psychosis. Proceedings of the XIII World Congress of Psychiatry; 2005 Sept 10‐15; Cairo, Egypt. Cairo, 2005.
Glen 1996 {published data only}
-
- Glen AIM, Cooper SJ, Rybakowski J, Vaddadi K, Brayshaw N, Horrobin DF. Membrane fatty acids, niacin flushing and clinical parameters. Prostaglandins, Leukotrienes and Essential Fatty Acids 1996;55:9‐15. - PubMed
Heresco‐Levy 1996 {published data only}
-
- Heresco Levy U, Javitt DC, Ermilov M, Mordel C, Horowitz A, Kelly D. Double‐blind, placebo‐controlled, crossover trial of glycine adjuvant therapy for treatment‐resistant schizophrenia. British Journal of Psychiatry 1996;169:610‐7. - PubMed
Holman 1983 {published data only}
-
- Holman CP, Bell AFJ. A trial of evening primrose oil in the treatment of chronic schizophrenia. Journal of Orthomolecular Psychiatry 1983;12:302‐4.
Maurer 2002 {published data only}
-
- Maurer I, Volz HP, Czekalla J, Holstein W, Streck S, Winnefel K. Reduction of arachidonic acid levels by haloperidol in patients with schizophrenia. Schizophrenia Research 2002;53(3 suppl 1):209.
Peet 1996 {published data only}
-
- Laugharne JDE, Mellor JE, Peet M. Fatty acids and schizophrenia. Lipids 1996;31:163‐5. - PubMed
-
- Mellor JE, Laugharne JDE, Peet M. Omega‐3 fatty acid supplementation in schizophrenic patients. Human Psychopharmacology 1996;11:39‐46.
-
- Mellor JE, Laugharne JDE, Peet M. Schizophrenic symptoms and dietary intake of n‐3 fatty acids. Schizophrenia Research 1995;18:85‐6. - PubMed
-
- Peet M, Laugharne JDE, Mellor J, Ramchand CN. Essential fatty acid deficiency in erythrocyte membranes from chronic schizophrenic patients, and the clinical effects of dietary supplementation. Prostaglandins, Leukotrienes and Essential Fatty Acids 1996;55:71‐5. - PubMed
Puri 2001 {published data only}
-
- Puri BK, Bydder GM, Counsell SJ, Corridan BJ, Richardson AJ, Hajnal JV, Appel C, McKee HM, Vaddadi KS, Horrobin DF. MRI and neuropsychological improvement in huntington disease following ethyl‐EPA treatment. Clinical Neuroscience 2002;13(1):123‐6. - PubMed
Silbergeld 1973 {published data only}
-
- Silbergeld S, Noble E. Corticosteroids in psychiatric patients: Subacute and diurnal effects on free fatty acid and catecholamine metabolism. Journal of Psychiatric Research 1973;10:59‐71. - PubMed
Vaddadi 1986 {published data only}
-
- Vaddadi KS, Gilleard CJ, Mindham RH, Butler R. A controlled trial of prostaglandin E1 precursor in chronic neuroleptic resistant schizophrenic patients. Psychopharmacology Berlin 1986;88:362‐7. - PubMed
Vaddadi 1989 {published data only}
-
- Vaddadi KS, Courtney P, Gilleard CJ, Manku MS, Horrobin DF. A double‐blind trial of essential fatty acid supplementation in patients with tardive dyskinesia. Psychiatry Research 1989;27:313‐23. - PubMed
-
- Vaddadi KS, Gilleard CJ. Essential fatty acids, tardive dyskinesia and schizophrenia. Omega‐6 Essential Fatty acids: Pathophysiology and Roles in Clinical Medicine. Alan R, Liss. Inc, 1990:333‐43.
References to ongoing studies
Bentsen 2007 {published data only}
-
- Bentsen H, Lingjaerde O. A multicentre, placebo‐controlled trial of eicosapentaenoic acid (EPA) and antioxidant supplementation in the treatment of schizophrenia and related disorders. www.clinicaltrials.gov 2007.
-
- Landro NI, Bentsen H. The effects of a fatty acid and antioxidants on neuropsychological functioning in schizophrenia and related psychoses. Schizophrenia Bulletin 2007;33:529‐30.
Blanchet 2008 {published data only}
-
- Blanchet PJ, Stip E. Efficacy of docosahexaenoic acid on tardive dyskinesia. www.clinicaltrials.gov 2008.
Korbanic 2005 {published data only}
-
- Korbanic CJ, Yao JK. CAD risk in schizophrenia: effect of omega‐3 fatty acid supplementation. www.clinicaltrials.gov 2005.
Murck 2001 {published data only}
-
- Murck H. A multicentre, double blind, randomised, parallel group, placebo controlled dose ranging pilot study of ethyl‐eicosapentaenoate (ethyl‐epa) in patients with schizophrenia. Current Controlled Trials 2001.
Richtand 2007 {published data only}
-
- Richtand N. Omega‐3 fatty acid deficiency replacement in early schizophrenia. www.clinicaltrials.gov 2007.
Additional references
Altman 1996
Begg 1996
-
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, Pitkin R, Rennie D, Schulz KF, Simel D, Stroup DFSO. Improving the quality of reporting of randomised controlled trials. The CONSORT statement [comment]. JAMA 1996;276:637‐9. - PubMed
Bland 1997
Christensen 1998
-
- Christensen O, Christensen E. Fat consumption and schizophrenia. Acta Psychiatrica Scandinavica 1998;78:587‐91. - PubMed
Di Marzo 1992
-
- Marzo V, Piomelli D. Participation of prostaglandin E2 in dopamine D2 receptor‐dependent potentiation of arachidonic response. Journal of Neurochemisty 1992;59:379‐82. - PubMed
Divine 1992
-
- Divine GW, Brown JT, Frazier LM. The unit of analysis error in studies about physicians' patient care behaviour. Journal of Internal Medicine 1992;7(6):623‐9. - PubMed
Donner 2002
-
- Donner A, Klar N. Issues in the meta‐analysis of cluster randomised trials. Statistics in Medicine 2002;21:2971‐80. - PubMed
Egger 1997
Elbourne 2002
-
- Elbourne D, Altman DG, Higgins JPT, Curtina F, Worthingtond HV, Vaile A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. - PubMed
Glen 1994
-
- Glen AIM, Glen EMT, Horrobin D, Vaddadi KS, Spellman M, Morse‐Fisher N, Ellisk, Skinner FS. A red cell membrane abnormality in a subgroup of schizophrenic patients: evidence for two diseases. Schizophrenia Research 1994;12:53‐61. - PubMed
Gulliford 1999
-
- Gulliford MC, Ukoumunne OC, Chinn S. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from Health Survey for England 1994. American Journal of Epidemiology 1999;149:876‐83. - PubMed
Guy 1976
-
- Guy Q. ECDEU assessment manual for psychopharmacology. ADM 76‐338. Rockville, MD: US Department of Health, Education and Welfare, 1976.
Heresco‐Levy 1998
-
- Heresco‐Levy U, Javitt DCAD. The role of N‐methyl‐D‐aspartate (NMDA) receptor‐mediated neurotransmission in the pathophysiology and therapeutics of psychiatric syndromes. European Neuropsychopharmacology 1998;8:141‐52. - PubMed
Higgins 2003
Higgins 2008
-
- Higgins, JPT, Green S (editors). Cochrane Handbook for systematic reviews of interventions 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008.
Horrobin 1991
-
- Horrobin DF, Manku MS, Hillman H, Iain A, Glen M. Fatty acid levels in the brains of schizophrenics and normal controls. Biological Psychiatry 1991;30:795‐805. - PubMed
Horrobin 1994
-
- Horrobin DF, Glen AIM, Vaddadi KSO. The membrane hypothesis of schizophrenia. Schizophrenia Research 1994;13:195‐207. - PubMed
Hunter 2003
Jablensky 1992
-
- Jablensky A, Sartorius N, Ernberg G, Anker M, Korten A, Cooper JE, Day R, Bertelsen A. Schizophrenia: manifestations, incidence and course in different cultures. A World Health Organization ten‐country study. Psychological Medicine Monograph Supplement 1992;20:1‐97. - PubMed
Kay 1986
-
- Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale(PANSS) manual. North Tonawanda (NY): Multi‐Health Systems, 1986.
Marshall 2000
-
- Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176:249‐52. - PubMed
Mellor 1995
-
- Mellor J, Laugharne JDE, Peet M. Schizophrenic symptoms and dietary intake of n‐3 fatty acids. Schizophrenia Research 1995;18:85‐6. - PubMed
Mellor 1996
-
- Mellor JE, Laugharne JDE, Peet M. Omega‐3 fatty acid supplementation in schizophrenic patients. Human Psychopharmacology 1996;11:39‐46.
Moher 2001
-
- Moher D, Schulz KF, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomised trials. JAMA 2001;285:1987‐91. - PubMed
Montgomery 1979
-
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134:382‐9. - PubMed
Nunez 1993
-
- Nunez EA, editor. Fatty acids and cell signalling. Prostaglandins Leukotrienes EFAs 1993;12:53‐61. - PubMed
Puri 1997
-
- Puri BK, Easton T, Richardson AJ. Normalisation of positive and negative symptoms of schizophrenia following dietary supplementation with essential fatty acids: a case study. Biological Psychiatry 1997;42:S189.
Simpson 1970
-
- Simpson GM, Angus JWS. A rating scale for extra‐pyramidal side‐effects. Acta Psychiatrica Scandinavica Supplementum 1970;212:11‐9. - PubMed
Ukoumunne 1999
-
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area‐wide and organisation‐based intervention in health and health care: a systematic review. Health Technology Assessment 1999;3(5):1‐75. - PubMed
WHO 1998
-
- WHO. Global Health Statistics Online. Available: http//www.who.ch/msa/mnh/ems/rates/schizo.htm. 24th July 1998.
Wiersma 1998
-
- Wiersma D, Nienhuis FJ, Slooff CJ, Giel R. Natural course of schizophrenic disorders: a 15‐year follow‐up of a Dutch incidence cohort. Schizophrenia Bulletin 1998;24:75‐85. - PubMed
Xia 2007
-
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El‐Sayeh H, Pinfold V, Takriti Y. The Leeds Outcomes Stakeholders Survey (LOSS) Study. Proceedings of the 15th Cochrane Colloquium; 2007 Oct 23‐27; Sao Paulo. 2007.
References to other published versions of this review
Joy 2000
-
- Joy CB, Mumby‐Croft R, Joy LA. Polyunsaturated fatty acid supplementation (fish or evening primrose oil) for schizophrenia: a Cochrane review. Schizophrenia Research 2000;41(1):27. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

