Medical treatment for early fetal death (less than 24 weeks)
- PMID: 16855990
- PMCID: PMC6464738
- DOI: 10.1002/14651858.CD002253.pub3
Medical treatment for early fetal death (less than 24 weeks)
Update in
-
Medical treatment for early fetal death (less than 24 weeks).Cochrane Database Syst Rev. 2019 Jun 17;6(6):CD002253. doi: 10.1002/14651858.CD002253.pub4. Cochrane Database Syst Rev. 2019. PMID: 31206170 Free PMC article.
Abstract
Background: In most pregnancies that miscarry, arrest of embryonic or fetal development occurs some time (often weeks) before the miscarriage occurs. Ultrasound examination can reveal abnormal findings during this phase by demonstrating anembryonic pregnancies or embryonic or fetal death. Treatment before 14 weeks has traditionally been surgical but medical treatments may be effective, safe, and acceptable, as may be waiting for spontaneous miscarriage.
Objectives: To assess the effectiveness, safety and acceptability of any medical treatment for early pregnancy failure (anembryonic pregnancies or embryonic and fetal deaths before 24 weeks).
Search strategy: We searched the Cochrane Pregnancy and Childbirth Group Trials Register (30 November 2005).
Selection criteria: Randomised trials comparing medical treatment with another treatment (e.g. surgical evacuation), or placebo, or no treatment for early pregnancy failure. Quasi-random studies were excluded.
Data collection and analysis: Data were extracted unblinded.
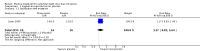
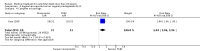
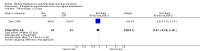
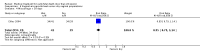
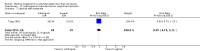
Main results: Twenty four studies (1888 women) were included. Vaginal misoprostol hastens miscarriage (complete or incomplete) when compared with placebo: e.g. miscarriage less than 24 hours (two trials, 138 women, relative risk (RR) 4.73, 95% confidence interval (CI) 2.70 to 8.28), with less need for uterine curettage (two trials, 104 women, RR 0.40, 95% CI 0.26 to 0.60) and no significant increase in nausea or diarrhoea. Lower-dose regimens of vaginal misoprostol tend to be less effective in producing miscarriage (three trials, 247 women, RR 0.85, 95% CI 0.72 to 1.00) with similar incidence of nausea. There seems no clear advantage to administering a 'wet' preparation of vaginal misoprostol or of adding methotrexate, or of using laminaria tents after 14 weeks. Vaginal misoprostol is more effective than vaginal prostaglandin E in avoiding surgical evacuation. Oral misoprostol was less effective than vaginal misoprostol in producing complete miscarriage (two trials, 218 women, RR 0.90, 95% CI 0.82 to 0.99). Sublingual misoprostol had equivalent efficacy to vaginal misoprostol in inducing complete miscarriage but was associated with more frequent diarrhoea. The two trials of mifepristone treatment generated conflicting results. There was no statistically significant difference between vaginal misoprostol and gemeprost in the induction of miscarriage for fetal death after 13 weeks.
Authors' conclusions: Available evidence from randomised trials supports the use of vaginal misoprostol as a medical treatment to terminate non-viable pregnancies before 24 weeks. Further research is required to assess effectiveness and safety, optimal route of administration and dose. Conflicting findings about the value of mifepristone need to be resolved by additional study.
Conflict of interest statement
None known.
Figures


















































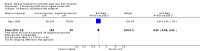



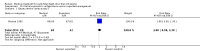






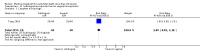



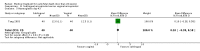










References
References to studies included in this review
-
- Al Inizi SA, Ezimokhai M. Vaginal misoprostol versus dinoprostone for the management of missed abortion. International Journal of Gynecology & Obstetrics 2003;83:73‐4. - PubMed
-
- Autry A, Jacobson G, Sandhu R, Isbill K. Medical management of non‐viable early first trimester pregnancy. International Journal of Gynecology & Obstetrics 1999;67(1):9‐13. - PubMed
-
- Bagratee JS, Khullar V, Regan L, Moodley J, Kagoro H. A randomized controlled trial comparing medical and expectant management of first trimester miscarriage. Human Reproduction 2004;19:266‐71. - PubMed
-
- Creinin MD, Moyer R, Guido R. Misoprostol for medical evacuation of early pregnancy failure. Obstetrics & Gynecology 1997;89:768‐72. - PubMed
-
- Demetroulis C, Saridogan E, Kunde D, Naftalin AA. A prospective randomized control trial comparing medical and surgical treatment for early pregnancy failure. Human Reproduction 2001;16:365‐9. - PubMed
- Demetroulis C, Saridogan E, Kunde D, Naftalin AA. A prospective randomized control trial comparing medical and surgical treatment for early pregnancy failure. XVI FIGO World Congress of Obstetrics and Gynecology; 2000 Sept 3‐8; Washington DC, USA. 2000. - PubMed
References to studies excluded from this review
-
- Abdel Fattah IH. PGE1 analogue for the induction of midtrimester abortion in cases of intrauterine fetal death. Acta Obstetricia et Gynecologica Scandinavica Supplement 1997;76(167:2):26.
-
- Almog B, Levin I, Winkler N, Fainaru O, Pauzner D, Lessing JB, et al. The contribution of laminaria placement for cervical ripening in second trimester termination of pregnancy induced by intra‐amniotic injection of prostaglandin F2alpha followed by concentrated oxytocin infusion. European Journal of Obstetrics & Gynecology and Reproductive Biology 2005;118:32‐5. - PubMed
-
- Anderman S, Jaschevatzky OE, Ballas S. Comparison between a double balloon device and the foley catheter in extraamniotic prostaglandin F2a infusion for termination of midtrimester missed abortion. XVI FIGO World Congress of Obstetrics & Gynecology; 2000 Sept 3‐8; Washington DC, USA. 2000:162.
-
- Avila‐Vergara MA, Morgan‐Ortiz F, Fragoza‐Sosa O, Haro‐Garcia L. Cervical labor induction with prostaglandin E2 in patients with fetal death [Maduracion cervical con prostaglandina E2 en pacientes con feto muerto]. Ginecologia y Obstetricia de Mexico 1997;65:155‐8. - PubMed
-
- Bebbington MW, Kent N, Lim K, Gagnon A, Delisle MF, Tessier F, et al. A randomized controlled trial comparing two protocols for the use of misoprostol in midtrimester pregnancy termination. American Journal of Obstetrics and Gynecology 2002;187:853‐7. - PubMed
References to studies awaiting assessment
-
- Henshaw RC, Hinshaw K, Smith NC, Templeton AA. The medical management of miscarriage. Fertility Society of Australia / Australian Gynaecological Endoscopy Society; 1995 November 19‐25; Melbourne, Australia. 1995:FSA 75.
- Hinshaw K, Rispin N, Smith N, Templeton A. Medical versus surgical management in first trimester miscarriage: a prospective, pragmatic random allocation trial. Journal of Obstetrics and Gynaecology 1993;13:404‐5.
- Hinshaw K, Rispin R, Henshaw R, Smith N, Templeton A. Medical versus surgical uterine evacuation in first trimester miscarriage: a prospective, pragmatic randomised trial. 27th British Congress of Obstetrics and Gynaecology; 1995 July 4‐7; Dublin. 1995:4.
- Hughes J, Ryan M, Hinshaw K, Henshaw R, Rispin R, Templeton A. The costs of treating miscarriage: a comparison of medical and surgical management. British Journal of Obstetrics and Gynaecology 1996;103:1217‐21. - PubMed
- Rispin R, Hinshaw K, Henshaw R, Smith N, Templeton A. New aspects of care in the management of miscarriage. Proceedings of Research in Midwifery Conference; 1993 September 14; Birmingham, UK. 1993.
-
- Al‐Bdour AN, Akasheh H, Al‐Jayousi T. Missed abortion: termination using single‐dose versus two doses of vaginal misoprostol tablets. Pakistan Journal of Medical Sciences 2007;23(6):920‐3.
-
- Altaf F, Sultana N, Iqbal N. Therapeutic abortions; efficacy of intra‐vaginal misoprostol in comparison to extra amniotically administered prostaglandin f2a. Professional Medical Journal 2006;13(3):417‐22.
-
- Anderson J, Gouk E, Young L, Turnbull L, Sayeed G, Elattar A, et al. A randomised controlled trial of oral versus vaginal misoprostol for medical management of early fetal demise. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S533.
-
- Ara G, Nargis S, Khatun R, Saha A. Vaginal misoprostol as a medical management in early pregnancy loss. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S533‐4.
References to ongoing studies
-
- Louey K. Misoprostol for the medical management of miscarriage (RCT). Personal communication2000.
-
- Trinder J. Miscarriage Treatment Study (MIST). National Research Register www.nrr.nhs.uk (accessed 1999).
Additional references
-
- Alfirevic Z. Oral misoprostol for induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 2. - PubMed
-
- Ashok PW, Penney GC, Flett GM, Templeton A. An effective regimen for early medical abortion: a report of 2000 consecutive cases. Human Reproduction 1998;13:2962‐5. - PubMed
-
- Baulieu E, Ulmann A. Antiprogesterone activity of RU‐486 and its contragestive and other applications. Human Reproduction 1986;1:107‐10. - PubMed
-
- Bugalho A, Faundes A, Jamisse L, Usfa M, Maria E, Bique C. Evaluation of the effectiveness of vaginal misoprostol to induce first trimester abortion. Contraception 1996;53:244‐6. - PubMed
-
- Cameron IT, Michie AF, Baird DT. Therapeutic abortion in early pregnancy with antiprogestogen RU486 alone or in combination with prostaglandin analogue (gemeprost). Contraception 1986;34(5):459‐68. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

