Drugs for preventing postoperative nausea and vomiting
- PMID: 16856030
- PMCID: PMC6463839
- DOI: 10.1002/14651858.CD004125.pub2
Drugs for preventing postoperative nausea and vomiting
Update in
-
WITHDRAWN: Drugs for preventing postoperative nausea and vomiting.Cochrane Database Syst Rev. 2017 Jul 17;7(7):CD004125. doi: 10.1002/14651858.CD004125.pub3. Cochrane Database Syst Rev. 2017. PMID: 28715610 Free PMC article.
Abstract
Background: Drugs can prevent postoperative nausea and vomiting, but their relative efficacies and side effects have not been compared within one systematic review.
Objectives: The objective of this review was to assess the prevention of postoperative nausea and vomiting by drugs and the development of any side effects.
Search strategy: We searched The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, Issue 2, 2004), MEDLINE (January 1966 to May 2004), EMBASE (January 1985 to May 2004), CINAHL (1982 to May 2004), AMED (1985 to May 2004), SIGLE (to May 2004), ISI WOS (to May 2004), LILAC (to May 2004) and INGENTA bibliographies.
Selection criteria: We included randomized controlled trials that compared a drug with placebo or another drug, or compared doses or timing of administration, that reported postoperative nausea or vomiting as an outcome.
Data collection and analysis: Two authors independently assessed trial quality and extracted outcome data.
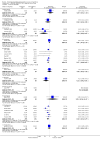
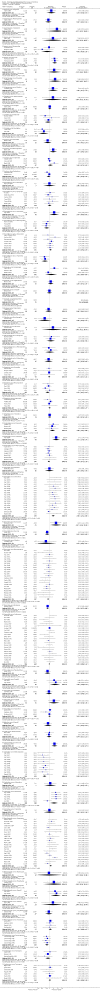
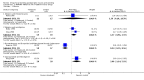
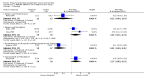
Main results: We included 737 studies involving 103,237 people. Compared to placebo, eight drugs prevented postoperative nausea and vomiting: droperidol, metoclopramide, ondansetron, tropisetron, dolasetron, dexamethasone, cyclizine and granisetron. Publication bias makes evidence for differences among these drugs unreliable. The relative risks (RR) versus placebo varied between 0.60 and 0.80, depending upon the drug and outcome. Evidence for side effects was sparse: droperidol was sedative (RR 1.32) and headache was more common after ondansetron (RR 1.16).
Authors' conclusions: Either nausea or vomiting is reported to affect, at most, 80 out of 100 people after surgery. If all 100 of these people are given one of the listed drugs, about 28 would benefit and 72 would not. Nausea and vomiting are usually less common and, therefore, drugs are less useful. For 100 people, of whom 30 would vomit or feel sick after surgery if given placebo, 10 people would benefit from a drug and 90 would not. Between one to five patients out of every 100 people may experience a mild side effect, such as sedation or headache, when given an antiemetic drug. Collaborative research should focus on determining whether antiemetic drugs cause more severe, probably rare, side effects. Further comparison of the antiemetic effect of one drug versus another is not a research priority.
Conflict of interest statement
None known.
Figures
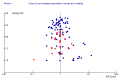
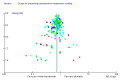
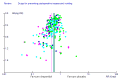
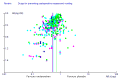















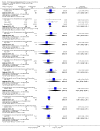
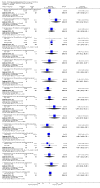

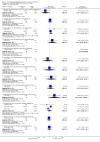
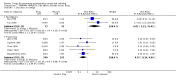





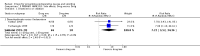












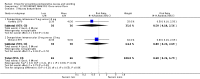
















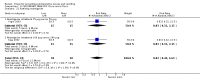

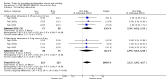

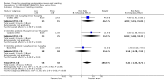









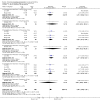













References
References to studies included in this review
-
- Aasboe V, Raeder JC, Groegaard B. Betamethasone reduces postoperative pain and nausea after ambulatory surgery. Anesthesia & Analgesia 1998 Aug;87(2):319‐23. [; MEDLINE: ] - PubMed
-
3219830
-
- Abdulatif M, Sanabary M. Caudal neostigmine, bupivacaine, and their combination for postoperative pain management after hypospadias surgery in children. Anesthesia & Analgesia 2002 Nov;95(5):1215‐8. [; MEDLINE: ; CN‐00410873] - PubMed
-
3219832
-
- Abou Zeid H, Al‐Gahamdi A, Abdul‐Hadi M. Dolasetron decreases postoperative nausea and vomiting after breast surgery. Breast Journal of Anaesthesia 2002 Jul;8(4):216‐21. [; MEDLINE: ; CN‐00390043] - PubMed
-
3219834
-
- Abramowitz MD, Oh TH, Epstein BS, Ruttimann UE, Friendly DS. The antiemetic effect of droperidol following outpatient strabismus surgery in children. Anesthesiology 1983 Dec;59(6):579‐83. [; MEDLINE: ] - PubMed
-
3219836
-
- Adducci E, Gorgoglione M, Aceto P, Congedo E, Clemente A, Gualtieri E, et al. The prophylaxis of postoperative nausea and vomiting after laparoscopic cholecystectomy. Acta Medica Romana 2002;40(4):331‐9. [; 2003425222 20031106 ISSN: 0001‐6098]
-
3219838
References to studies excluded from this review
-
- Abouleish EI, Rashid S, Haque S, Giezentanner A, Joynton P, Chuang AZ. Ondansetron versus placebo for the control of nausea and vomiting during Caesarean section under spinal anaesthesia. Anaesthesia 1999 May;54(5):479‐82. [; MEDLINE: ] - PubMed
-
3221304
-
- Alexander R, Fennelly M. Comparison of ondansetron and metoclopramide as premedicants to prevent postoperative nausea and vomiting following orthopedic‐ surgery. British Journal of Anaesthesia 1995 May;74:91. [; ISI:A1995RA77700296 ER]
-
3221306
-
- Alon E, Zamboni P, Hossli G. Postoperative antiemetic prophylaxis with haloperidol and metoclopramid in differential dosages. Schweizerische medizinische Wochenschrift 1987 Mar;17(12):460. [; ISI:A1987G525100031 ER]
-
3221308
-
- Alon E, Lenzlinger PM, Pasch T. Ondansetron 4 mg vs droperidol 1.25 mg in the prophylaxis of postoperative nausea and vomiting after alfentanil‐supplemented inhalation anesthesia. British journal of anaesthesia 1993 May;70:6. [; ISI:A1993LD22600012 ER]
-
3221310
-
- Ambesh SP, Dubey PK, Sinha PK. Ondansetron pretreatment to alleviate pain on propofol injection: a randomized, controlled, double‐blinded study. Anesthesia & Analgesia 1999 Jul;89(1):197‐9. [; MEDLINE: ] - PubMed
-
3221312
Additional references
-
- Apfel CC, Kranke P, Greim CA, Roewer N. Non‐systematic serial publishing is not appropriate and ethically questionable. Acta Anaesthesiologica Scandinivica 1999;43(4):486‐7. [MEDLINE: ] - PubMed
-
- Apfel CC, Kranke P, Eberhart LHJ, Roos A, Roewer N. Comparison of predictive models for postoperative nausea and vomiting. British Journal of Anaesthesia 2002;88(2):234‐40. - PubMed
-
- Cohen MM, Duncan PG, DeBoer DP, Tweed WA. The postoperative interview: assessing risk factors for nausea and vomiting. Anesthesia and Analgesia 1994;78:7‐16. - PubMed
-
- Eberhart LH, Mauch M, Morin AM, Wulf H, Geldner G. Impact of a multimodal anti‐emetic prophylaxis on patient satisfaction in high‐risk patients for postoperative nausea and vomiting. Anaesthesia 2002 Oct;57(10):1022‐7. [MEDLINE: ] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

