Toxicogenomics in regulatory ecotoxicology
- PMID: 16856717
- PMCID: PMC1892581
- DOI: 10.1021/es0630184
Toxicogenomics in regulatory ecotoxicology
Figures



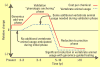
References
-
- Nuwaysir EF, et al. Microarrays and Toxicology: The Advent of Toxicogenomics. Mol Carcinogen. 1999;24:153–159. - PubMed
-
- MacGregor JT. The Future of Regulatory Toxicology: Impact of the Biotechnology Revolution. Toxicol Sci. 2003;75:236–248. - PubMed
-
- Waters MD, Fostel JM. Toxicogenomics and Systems Toxicology: Aims and Prospects. Nat Rev Genet. 2004;5:936–948. - PubMed
-
- Bilello JA. The Agony and Ecstasy of “OMIC” Technologies in Drug Development. Curr Mol Med. 2005;5:39–52. - PubMed
-
- Lühe A, et al. Toxicogenomics in the Pharmaceutical Industry: Hollow Promises or Real Benefit? Mutat Res. 2005;575:102–115. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

