Structural and kinetic analyses of the H121A mutant of cholesterol oxidase
- PMID: 16856877
- PMCID: PMC1635447
- DOI: 10.1042/BJ20060664
Structural and kinetic analyses of the H121A mutant of cholesterol oxidase
Abstract
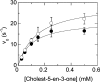
Cholesterol oxidase is a monomeric flavoenzyme that catalyses the oxidation of cholesterol to cholest-5-en-3-one followed by isomerization to cholest-4-en-3-one. The enzyme from Brevibacterium sterolicum contains the FAD cofactor covalently bound to His121. It was previously demonstrated that the H121A substitution results in a approximately 100 mV decrease in the midpoint redox potential and a approximately 40-fold decrease in turnover number compared to wild-type enzyme [Motteran, Pilone, Molla, Ghisla and Pollegioni (2001) Journal of Biological Chemistry 276, 18024-18030]. A detailed kinetic analysis of the H121A mutant enzyme shows that the decrease in turnover number is largely due to a corresponding decrease in the rate constant of flavin reduction, whilst the re-oxidation reaction is only marginally altered and the isomerization reaction is not affected by the substitution and precedes product dissociation. The X-ray structure of the mutant protein, determined to 1.7 A resolution (1 A identical with 0.1 nm), reveals only minor changes in the overall fold of the protein, namely: two loops have slight movements and a tryptophan residue changes conformation by a rotation of 180 degrees about chi1 compared to the native enzyme. Comparison of the isoalloxazine ring moiety of the FAD cofactor between the structures of the native and mutant proteins shows a change from a non-planar to a planar geometry (resulting in a more tetrahedral-like geometry for N5). This change is proposed to be a major factor contributing to the observed alteration in redox potential. Since a similar distortion of the flavin has not been observed in other covalent flavoproteins, it is proposed to represent a specific mode to facilitate flavin reduction in covalent cholesterol oxidase.
Figures

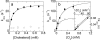



References
-
- MacLachlan J., Wotherspoon A. T. L., Ansell R. O., Brooks C. J. W. Cholesterol oxidase: sources, physical properties and analytical applications. J. Steroid Biochem. Mol. Biol. 2000;72:169–195. - PubMed
-
- Corbin D. R., Greenplate J. T., Purcell J. P. The identification and development of proteins for control of insects in genetically modified crops. HortScience. 1998;33:614–617.
-
- Li J., Vrielink A., Brick P., Blow D. M. Crystal structure of cholesterol oxidase complexed with a steroid substrate: Implications for flavin adenine dinucleotide dependent alcohol oxidases. Biochemistry. 1993;32:11507–11515. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

