The effects of human CYP2C8 genotype and fluvoxamine on the pharmacokinetics of rosiglitazone in healthy subjects
- PMID: 16856883
- PMCID: PMC1885187
- DOI: 10.1111/j.1365-2125.2006.02706.x
The effects of human CYP2C8 genotype and fluvoxamine on the pharmacokinetics of rosiglitazone in healthy subjects
Abstract
Aims: To determine the effect of CYP2C8 genotype and of fluvoxamine on the pharmacokinetics of rosiglitazone.
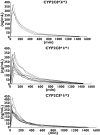
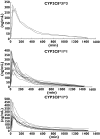
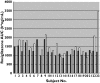
Methods: Twenty-three healthy subjects with the following genotypes were included in a two-phase, open-label, cross-over trial: CYP2C8*3/ *3 (n = 3), CYP2C8*1/ *3 (n = 10) and CYP2C8*1/ *1 (n = 10). In Phase A, the subjects were given 4 mg rosiglitazone as a single oral dose. In Phase B, the subjects were treated with multiple oral doses of 50 mg fluvoxamine maleate for 3 days prior to the single oral administration of 4 mg rosiglitazone. Plasma concentrations of rosiglitazone and relative amounts of N-desmethylrosiglitazone were measured in both phases for 24 h after drug administration.
Results: The pharmacokinetics of rosiglitazone and N-desmethylrosiglitazone were not significantly different between the CYP2C8 genotypic groups. Fluvoxamine caused a statistically significant (P = 0.0066) increase in the AUC(0-infinity) of rosiglitazone, with a geometric mean ratio of 1.21 [95% confidence interval (CI) 1.06-1.39]. The elimination half-life (t(1/2)) was also significantly higher (P = 0.0203) with a geometric mean ratio of 1.38 [95% CI 1.06-1.79]. The coadministration of fluvoxamine had no influence on the pharmacokinetics of N-desmethylrosiglitazone.
Conclusion: The importance of the CYP2C8*3 mutation in the in vivo metabolism of rosiglitazone could not be confirmed. Fluvoxamine increased the AUC(0-infinity) and t(1/2) of rosiglitazone moderately and hence may be a weak inhibitor of CYP2C8.
Figures
References
-
- Patel J, Miller E, Patwardhan R. Rosiglitazone (BRL49653) monotherapy has significant glucose lowering effect in type 2 diabetic patients. Diabetes. 1998;47:A17.
-
- Patel J, Anderson RJ, Rappaport EB. Rosiglitazone monotherapy improves glycaemic control in patients with type 2 diabetes: a twelve-week, randomized, placebo-controlled study. Diabetes Obes Metab. 1999;1:165–72. - PubMed
-
- Young PW, Buckle DR, Cantello BCC, Chapman H, Clapham JC, Coyle PJ, Haigh D, Hindley RM, Holder JC, Kallender H, Latter AJ, Lawrie KWM, Mossakowska D, Murphy GJ, Cox LR, Smith S. Identification of high-affinity binding sites for the insulin sensitizer rosiglitazone (BRL-49653) in rodent and human adipocytes using a radioiodinated ligand for peroxisomal proliferator-activated receptor gamma. J Pharmacol Exp Ther. 1998;284:751–9. - PubMed
-
- Cox PJ, Ryan DA, Hollis FJ, Harris AM, Miller AK, Vousden M, Cowley H. Absorption, disposition, and metabolism of rosiglitazone, a potent thiazolidinedione insulin sensitizer, in humans. Drug Metab Dispos. 2000;28:772–80. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

