Expression of mammalian GPCRs in C. elegans generates novel behavioural responses to human ligands
- PMID: 16857046
- PMCID: PMC1550261
- DOI: 10.1186/1741-7007-4-22
Expression of mammalian GPCRs in C. elegans generates novel behavioural responses to human ligands
Abstract
Background: G-protein-coupled receptors (GPCRs) play a crucial role in many biological processes and represent a major class of drug targets. However, purification of GPCRs for biochemical study is difficult and current methods of studying receptor-ligand interactions involve in vitro systems. Caenorhabditis elegans is a soil-dwelling, bacteria-feeding nematode that uses GPCRs expressed in chemosensory neurons to detect bacteria and environmental compounds, making this an ideal system for studying in vivo GPCR-ligand interactions. We sought to test this by functionally expressing two medically important mammalian GPCRs, somatostatin receptor 2 (Sstr2) and chemokine receptor 5 (CCR5) in the gustatory neurons of C. elegans.
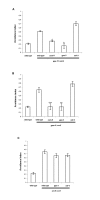
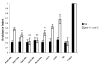
Results: Expression of Sstr2 and CCR5 in gustatory neurons allow C. elegans to specifically detect and respond to somatostatin and MIP-1alpha respectively in a robust avoidance assay. We demonstrate that mammalian heterologous GPCRs can signal via different endogenous Galpha subunits in C. elegans, depending on which cells it is expressed in. Furthermore, pre-exposure of GPCR transgenic animals to its ligand leads to receptor desensitisation and behavioural adaptation to subsequent ligand exposure, providing further evidence of integration of the mammalian GPCRs into the C. elegans sensory signalling machinery. In structure-function studies using a panel of somatostatin-14 analogues, we identified key residues involved in the interaction of somatostatin-14 with Sstr2.
Conclusion: Our results illustrate a remarkable evolutionary plasticity in interactions between mammalian GPCRs and C. elegans signalling machinery, spanning 800 million years of evolution. This in vivo system, which imparts novel avoidance behaviour on C. elegans, thus provides a simple means of studying and screening interaction of GPCRs with extracellular agonists, antagonists and intracellular binding partners.
Figures

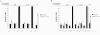
Similar articles
-
Control of feeding behavior in C. elegans by human G protein-coupled receptors permits screening for agonist-expressing bacteria.Proc Natl Acad Sci U S A. 2008 Sep 30;105(39):14826-31. doi: 10.1073/pnas.0803290105. Epub 2008 Sep 24. Proc Natl Acad Sci U S A. 2008. PMID: 18815363 Free PMC article.
-
Functional expression of mammalian bitter taste receptors in Caenorhabditis elegans.Biochimie. 2006 Jul;88(7):801-6. doi: 10.1016/j.biochi.2006.01.008. Epub 2006 Feb 9. Biochimie. 2006. PMID: 16494987
-
Global analysis of neuropeptide receptor conservation across phylum Nematoda.BMC Biol. 2024 Oct 8;22(1):223. doi: 10.1186/s12915-024-02017-6. BMC Biol. 2024. PMID: 39379997 Free PMC article.
-
The role of GPCR dimerisation/oligomerisation in receptor signalling.Ernst Schering Found Symp Proc. 2006;(2):145-61. doi: 10.1007/2789_2006_007. Ernst Schering Found Symp Proc. 2006. PMID: 17703581 Review.
-
GPCR Signaling in C. elegans and Its Implications in Immune Response.Adv Immunol. 2017;136:203-226. doi: 10.1016/bs.ai.2017.05.002. Epub 2017 Jun 23. Adv Immunol. 2017. PMID: 28950946 Review.
Cited by
-
The interplay of helminthic neuropeptides and proteases in parasite survival and host immunomodulation.Biochem Soc Trans. 2022 Feb 28;50(1):107-118. doi: 10.1042/BST20210405. Biochem Soc Trans. 2022. PMID: 35076687 Free PMC article. Review.
-
Control of feeding behavior in C. elegans by human G protein-coupled receptors permits screening for agonist-expressing bacteria.Proc Natl Acad Sci U S A. 2008 Sep 30;105(39):14826-31. doi: 10.1073/pnas.0803290105. Epub 2008 Sep 24. Proc Natl Acad Sci U S A. 2008. PMID: 18815363 Free PMC article.
-
Transcriptome-Based Analysis Reveals a Crucial Role of BxGPCR17454 in Low Temperature Response of Pine Wood Nematode (Bursaphelenchus xylophilus).Int J Mol Sci. 2019 Jun 14;20(12):2898. doi: 10.3390/ijms20122898. Int J Mol Sci. 2019. PMID: 31197083 Free PMC article.
-
Trapping the nematode on a micro chip for the future of science.HFSP J. 2007 Nov;1(4):220-4. doi: 10.2976/1.2806028. Epub 2007 Nov 29. HFSP J. 2007. PMID: 19404422 Free PMC article.
-
Pharmacological Profiling of a Brugia malayi Muscarinic Acetylcholine Receptor as a Putative Antiparasitic Target.Antimicrob Agents Chemother. 2023 Jan 24;67(1):e0118822. doi: 10.1128/aac.01188-22. Epub 2023 Jan 5. Antimicrob Agents Chemother. 2023. PMID: 36602350 Free PMC article.
References
-
- Bargmann CI, Mori I. Chemotaxis and thermotaxis. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editor. C elegans II. Vol. 25. Cold Spring Harbour, NY: Cold Spring Harbour Laboratory Press; 1997. pp. 717–737. - PubMed
-
- Milani N, Guarin E, Renfer E, Nef P, Andres-Barquin PJ. Functional expression of a mammalian olfactory receptor in Caenorhabditis elegans. Chem Neurosci. 2002;13:2515–2520. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

