Regulation of the desensitization and ion selectivity of ATP-gated P2X2 channels by phosphoinositides
- PMID: 16857707
- PMCID: PMC1995631
- DOI: 10.1113/jphysiol.2006.115246
Regulation of the desensitization and ion selectivity of ATP-gated P2X2 channels by phosphoinositides
Abstract
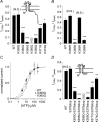
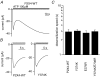
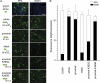
Phosphoinositides (PIP(n)s) are known to regulate the activity of some ion channels. Here we determined that ATP-gated P2X(2) channels also are regulated by PIP(n)s, and investigated the structural background and the unique features of this regulation. We initially used two-electrode voltage clamp to analyse the electrophysiological properties of P2X(2) channels expressed in Xenopus oocytes, and observed that preincubation with wortmannin or LY294002, two PI3K inhibitors, accelerated channel desensitization. K365Q or K369Q mutation of the conserved, positively charged, amino acid residues in the proximal region of the cytoplasmic C-terminal domain also accelerated desensitization, whereas a K365R or K369R mutation did not. We observed that the permeability of the channel to N-methyl-d-glucamine (NMDG) transiently increased and then decreased after ATP application, and that the speed of the decrease was accelerated by K365Q or K369Q mutation or PI3K inhibition. Using GST-tagged recombinant proteins spanning the proximal C-terminal region, we then analysed their binding of the P2X(2) cytoplasmic domain to anionic lipids using PIP(n)s-coated nitrocellulose membranes. We found that the recombinant proteins that included the positively charged region bound to PIPs and PIP(2)s, and that this binding was eliminated by the K365Q and K369Q mutations. We also used a fluorescence assay to confirm that fusion proteins comprising the proximal C-terminal region of P2X(2) with EGFP expressed in COS-7 cells closely associated with the membrane. Taken together, these results show that membrane-bound PIP(n)s play a key role in maintaining channel activity and regulating pore dilation through electrostatic interaction with the proximal region of the P2X(2) cytoplasmic C-terminal domain.
Figures






References
-
- Baukrowitz T, Schulte U, Oliver D, Herlitze S, Krauter T, Tucker SJ, Ruppersberg JP, Fakler B. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science. 1998;282:1141–1144. - PubMed
-
- Brake AJ, Wagenbach MJ, Julius D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature. 1994;371:519–523. - PubMed
-
- Chaumont S, Jiang LH, Penna A, North RA, Rassendren F. Identification of a trafficking motif involved in the stabilization and polarization of P2X receptors. J Biol Chem. 2004;279:29628–29638. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

