Alternative splicing in the C-terminus of CaV2.2 controls expression and gating of N-type calcium channels
- PMID: 16857708
- PMCID: PMC1995641
- DOI: 10.1113/jphysiol.2006.115030
Alternative splicing in the C-terminus of CaV2.2 controls expression and gating of N-type calcium channels
Abstract
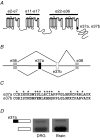
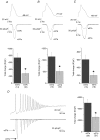
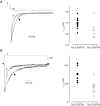
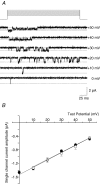
N-type Ca(V)2.2 calcium channels localize to presynaptic nerve terminals of nociceptors where they control neurotransmitter release. Nociceptive neurons express a unique set of ion channels and receptors important for optimizing their role in transmission of noxious stimuli. Included among these is a structurally and functionally distinct N-type calcium channel splice isoform, Ca(V)2.2e[37a], expressed in a subset of nociceptors and with limited expression in other parts of the nervous system. Ca(V)2.2[e37a] arises from the mutually exclusive replacement of e37a for e37b in the C-terminus of Ca(V)2.2 mRNA. N-type current densities in nociceptors that express a combination of Ca(V)2.2e[37a] and Ca(V)2.2e[37b] mRNAs are significantly larger compared to cells that express only Ca(V)2.2e[37b]. Here we show that e37a supports increased expression of functional N-type channels and an increase in channel open time as compared to Ca(V)2.2 channels that contain e37b. To understand how e37a affects N-type currents we compared macroscopic and single-channel ionic currents as well as gating currents in tsA201 cells expressing Ca(V)2.2e[37a] and Ca(V)2.2e[37b]. When activated, Ca(V)2.2e[37a] channels remain open for longer and are expressed at higher density than Ca(V)2.2e[37b] channels. These unique features of the Ca(V)2.2e[37a] isoform combine to augment substantially the amount of calcium that enters cells in response to action potentials. Our studies of the e37a/e37b splice site reveal a multifunctional domain in the C-terminus of Ca(V)2.2 that regulates the overall activity of N-type calcium channels in nociceptors.
Figures




Comment in
-
Alternative splicing of the Cav2.2 subunit: a change in N-type calcium channel activity for which purpose?J Physiol. 2007 Apr 15;580(Pt. 2):361-2. doi: 10.1113/jphysiol.2007.129130. Epub 2007 Feb 22. J Physiol. 2007. PMID: 17317741 Free PMC article. No abstract available.
Similar articles
-
Neuronal calcium channels: splicing for optimal performance.Cell Calcium. 2007 Oct-Nov;42(4-5):409-17. doi: 10.1016/j.ceca.2007.04.003. Epub 2007 May 18. Cell Calcium. 2007. PMID: 17512586 Free PMC article. Review.
-
Cacna1b alternative splicing impacts excitatory neurotransmission and is linked to behavioral responses to aversive stimuli.Mol Brain. 2019 Oct 21;12(1):81. doi: 10.1186/s13041-019-0500-1. Mol Brain. 2019. PMID: 31630675 Free PMC article.
-
Alternative splicing matters: N-type calcium channels in nociceptors.Channels (Austin). 2007 Jul-Aug;1(4):225-7. doi: 10.4161/chan.4809. Epub 2007 Jul 30. Channels (Austin). 2007. PMID: 18708749
-
Differential regulation of Cav2.2 channel exon 37 variants by alternatively spliced μ-opioid receptors.Mol Brain. 2019 Nov 27;12(1):98. doi: 10.1186/s13041-019-0524-6. Mol Brain. 2019. PMID: 31775826 Free PMC article.
-
The neuronal splicing factor Nova controls alternative splicing in N-type and P-type CaV2 calcium channels.Channels (Austin). 2010 Nov-Dec;4(6):483-9. doi: 10.4161/chan.4.6.12868. Epub 2010 Nov 1. Channels (Austin). 2010. PMID: 21150296 Free PMC article. Review.
Cited by
-
Cell-specific exon methylation and CTCF binding in neurons regulate calcium ion channel splicing and function.Elife. 2020 Mar 26;9:e54879. doi: 10.7554/eLife.54879. Elife. 2020. PMID: 32213287 Free PMC article.
-
Neuronal calcium channels: splicing for optimal performance.Cell Calcium. 2007 Oct-Nov;42(4-5):409-17. doi: 10.1016/j.ceca.2007.04.003. Epub 2007 May 18. Cell Calcium. 2007. PMID: 17512586 Free PMC article. Review.
-
Role of voltage-gated calcium channels in ascending pain pathways.Brain Res Rev. 2009 Apr;60(1):84-9. doi: 10.1016/j.brainresrev.2008.12.021. Epub 2008 Dec 31. Brain Res Rev. 2009. PMID: 19162069 Free PMC article. Review.
-
Cacna1b alternative splicing impacts excitatory neurotransmission and is linked to behavioral responses to aversive stimuli.Mol Brain. 2019 Oct 21;12(1):81. doi: 10.1186/s13041-019-0500-1. Mol Brain. 2019. PMID: 31630675 Free PMC article.
-
Voltage-gated calcium channels in chronic pain: emerging role of alternative splicing.Pflugers Arch. 2008 Jun;456(3):459-66. doi: 10.1007/s00424-007-0390-4. Epub 2008 Apr 4. Pflugers Arch. 2008. PMID: 18389277 Review.
References
-
- Agler HL, Evans J, Tay LH, Anderson MJ, Colecraft HM, Yue DT. G protein-gated inhibitory module of N-type (ca(v)2.2) ca2+ channels. Neuron. 2005;46:891–904. - PubMed
-
- Altier C, Khosravani H, Evans RM, Hameed S, Peloquin JB, Vartian BA, et al. ORL1 receptor-mediated internalization of N-type calcium channels. Nat Neurosci. 2006;9:31–40. - PubMed
-
- Altier C, Zamponi GW. Targeting Ca2+ channels to treat pain: T-type versus N-type. Trends Pharmacol Sci. 2004;25:465–470. - PubMed
-
- Bell TJ, Thaler C, Castiglioni AJ, Helton TD, Lipscombe D. Cell-specific alternative splicing increases calcium channel current density in the pain pathway. Neuron. 2004;41:127–138. - PubMed
-
- Bezanilla F. The voltage sensor in voltage-dependent ion channels. Physiol Rev. 2000;80:555–592. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

