Early chronic ethanol exposure in rats disturbs respiratory network activity and increases sensitivity to ethanol
- PMID: 16857714
- PMCID: PMC1995622
- DOI: 10.1113/jphysiol.2006.111138
Early chronic ethanol exposure in rats disturbs respiratory network activity and increases sensitivity to ethanol
Abstract
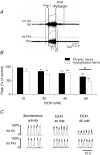
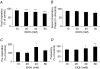
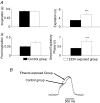
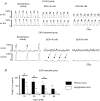
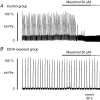
Chronic ethanol exposure during the fetal period alters spontaneous neuronal discharge, excitatory and inhibitory amino acid neurotransmission and neuronal sensitivity to ethanol in the adult brain. However, nothing is known about the effects of such exposure on the central respiratory rhythmic network, which is highly dependent on ethanol-sensitive amino acid neurotransmission. In 3- to 4-week-old rats, we investigated (1) the effects of chronic ethanol exposure (10% v/v as only source of fluid) during gestation and lactation on phrenic (Phr) and hypoglossal (XII) nerve activity using an in situ preparation and on spontaneous breathing at rest in unanaesthetized animals using plethysmography; (2) the sensitivity of the respiratory system to ethanol re-exposure in situ; and (3) the phrenic nerve response to muscimol, a GABA(A) receptor agonist, applied systemically in an in situ preparation. In control rats, ethanol (10-80 mm) induced a concentration-dependent decrease in the amplitude of both XII and Phr motor outflows. At 80 mm ethanol, the amplitude of the activity of the two nerves displayed a difference in sensitivity to ethanol and respiratory frequency increased as a result of shortening of postinspiratory duration period. After chronic ethanol exposure, respiratory frequency was significantly reduced by 43% in situ and by 23% in unanaesthetized animals, as a result of a selective increase in expiratory duration. During Phr burst, the ramp was steeper, revealing modification of inspiratory patterning. Interestingly that re-exposure to ethanol in situ elicited a dramatic inhibitory effect. At 80 mm, ethanol abolished rhythmic XII nerve outflow in all cases and Phr nerve outflow in only 50% of cases. Furthermore, administration of 50 microm muscimol abolished Phr nerve activity in all control rats, but only in 50% of ethanol-exposed animals. Our results demonstrate that chronic ethanol exposure at an early stage of brain development depresses breathing in juvenile rats, and sensitizes the respiratory network to re-exposure to ethanol, which does not seem to involve GABAergic neurotransmission.
Figures
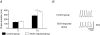
Similar articles
-
Blunted response to low oxygen of rat respiratory network after perinatal ethanol exposure: involvement of inhibitory control.J Physiol. 2008 Mar 1;586(5):1413-27. doi: 10.1113/jphysiol.2007.147165. Epub 2007 Dec 20. J Physiol. 2008. PMID: 18096598 Free PMC article.
-
Biphasic effect of acamprosate on NMDA but not on GABAA receptors in spontaneous rhythmic activity from the isolated neonatal rat respiratory network.Neuropharmacology. 2004 Jul;47(1):35-45. doi: 10.1016/j.neuropharm.2004.03.004. Neuropharmacology. 2004. PMID: 15165832
-
The effects of NMDA and GABAA pharmacological manipulations on ethanol sensitivity in immature and mature animals.Alcohol Clin Exp Res. 2002 Apr;26(4):449-56. Alcohol Clin Exp Res. 2002. PMID: 11981119
-
A conceptualization of integrated actions of ethanol contributing to its GABAmimetic profile: a commentary.Neuropsychopharmacology. 2005 Aug;30(8):1407-25. doi: 10.1038/sj.npp.1300750. Neuropsychopharmacology. 2005. PMID: 15856077 Review.
-
Inactivity-induced respiratory plasticity: protecting the drive to breathe in disorders that reduce respiratory neural activity.Respir Physiol Neurobiol. 2013 Nov 1;189(2):384-94. doi: 10.1016/j.resp.2013.06.023. Epub 2013 Jun 28. Respir Physiol Neurobiol. 2013. PMID: 23816599 Free PMC article. Review.
Cited by
-
Neurodevelopmental alcohol exposure elicits long-term changes to gene expression that alter distinct molecular pathways dependent on timing of exposure.J Neurodev Disord. 2013 Mar 13;5(1):6. doi: 10.1186/1866-1955-5-6. J Neurodev Disord. 2013. PMID: 23497526 Free PMC article.
-
Effect of chronic ethanol exposure on rat ventilatory responses to hypoxia and hypercapnia.Clinics (Sao Paulo). 2014;69(5):360-6. doi: 10.6061/clinics/2014(05)11. Clinics (Sao Paulo). 2014. PMID: 24838903 Free PMC article.
-
Moderate ethanol exposure during early ontogeny of the rat alters respiratory plasticity, ultrasonic distress vocalizations, increases brain catalase activity, and acetaldehyde-mediated ethanol intake.Front Behav Neurosci. 2022 Nov 10;16:1031115. doi: 10.3389/fnbeh.2022.1031115. eCollection 2022. Front Behav Neurosci. 2022. PMID: 36439967 Free PMC article.
-
Fetal alcohol-induced hyperactivity is reversed by treatment with the PPARα agonist fenofibrate in a rat model.Psychopharmacology (Berl). 2011 Mar;214(1):285-96. doi: 10.1007/s00213-010-1960-2. Epub 2010 Jul 27. Psychopharmacology (Berl). 2011. PMID: 20661551
-
Blunted response to low oxygen of rat respiratory network after perinatal ethanol exposure: involvement of inhibitory control.J Physiol. 2008 Mar 1;586(5):1413-27. doi: 10.1113/jphysiol.2007.147165. Epub 2007 Dec 20. J Physiol. 2008. PMID: 18096598 Free PMC article.
References
-
- Abel EL, Berman RF. Long-term behavioral effects of prenatal alcohol exposure in rats. Neurotoxicol Teratol. 1994;16:467–470. - PubMed
-
- Allan AM, Wu H, Paxton LL, Savage DD. Prenatal ethanol exposure alters the modulation of the gamma-aminobutyric acidA1 receptor-gated chloride ion channel in adult rat offspring. J Pharmacol Exp Ther. 1998;284:250–257. - PubMed
-
- Bartlett D, Jr, Tenney SM. Control of breathing in experimental anemia. Respir Physiol. 1970;10:384–395. - PubMed
-
- Bianchi AL, Denavit-Saubie M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev. 1995;75:1–45. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

