The flounder organic anion transporter fOat has sequence, function, and substrate specificity similarity to both mammalian Oat1 and Oat3
- PMID: 16857889
- PMCID: PMC1832143
- DOI: 10.1152/ajpregu.00326.2006
The flounder organic anion transporter fOat has sequence, function, and substrate specificity similarity to both mammalian Oat1 and Oat3
Abstract
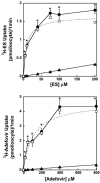
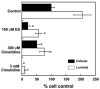
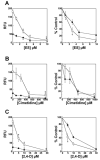
The flounder renal organic anion transporter (fOat) has substantial sequence homology to mammalian basolateral organic anion transporter orthologs (OAT1/Oat1 and OAT3/Oat3), suggesting that fOat may have functional properties of both mammalian forms. We therefore compared uptake of various substrates by rat Oat1 and Oat3 and human OAT1 and OAT3 with the fOat clone expressed in Xenopus oocytes. These data confirm that estrone sulfate is an excellent substrate for mammalian OAT3/Oat3 transporters but not for OAT1/Oat1 transporters. In contrast, 2,4-dichlorophenoxyacetic acid and adefovir are better transported by mammalian OAT1/Oat1 than by the OAT3/Oat3 clones. All three substrates were well transported by fOat-expressing Xenopus oocytes. fOat K(m) values were comparable to those obtained for mammalian OAT/Oat1/3 clones. We also characterized the ability of these substrates to inhibit uptake of the fluorescent substrate fluorescein in intact teleost proximal tubules isolated from the winter flounder (Pseudopleuronectes americanus) and killifish (Fundulus heteroclitus). The rank order of the IC(50) values for inhibition of cellular fluorescein accumulation was similar to that for the K(m) values obtained in fOat-expressing oocytes, suggesting that fOat may be the primary teleost renal basolateral Oat. Assessment of the zebrafish (Danio rerio) genome indicated the presence of a single Oat (zfOat) with similarity to both mammalian OAT1/Oat1 and OAT3/Oat3. The puffer fish (Takifugu rubripes) also has an Oat (pfOat) similar to mammalian OAT1/Oat1 and OAT3/Oat3 members. Furthermore, phylogenetic analyses argue that the teleost Oat1/3-like genes diverged from a common ancestral gene in advance of the divergence of the mammalian OAT1/Oat1, OAT3/Oat3, and, possibly, Oat6 genes.
Figures
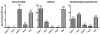


Similar articles
-
Differential interaction of dicarboxylates with human sodium-dicarboxylate cotransporter 3 and organic anion transporters 1 and 3.Am J Physiol Renal Physiol. 2011 Nov;301(5):F1026-34. doi: 10.1152/ajprenal.00169.2011. Epub 2011 Aug 24. Am J Physiol Renal Physiol. 2011. PMID: 21865262
-
The molecular and cellular physiology of basolateral organic anion transport in mammalian renal tubules.Biochim Biophys Acta. 2003 Dec 30;1618(2):185-93. doi: 10.1016/j.bbamem.2003.08.015. Biochim Biophys Acta. 2003. PMID: 14729155 Review.
-
Zebrafish (Danio rerio) Oat1 and Oat3 transporters and their interaction with physiological compounds.Comp Biochem Physiol B Biochem Mol Biol. 2019 Oct;236:110309. doi: 10.1016/j.cbpb.2019.110309. Epub 2019 Jun 27. Comp Biochem Physiol B Biochem Mol Biol. 2019. PMID: 31255699
-
A role for the organic anion transporter OAT3 in renal creatinine secretion in mice.Am J Physiol Renal Physiol. 2012 May 15;302(10):F1293-9. doi: 10.1152/ajprenal.00013.2012. Epub 2012 Feb 15. Am J Physiol Renal Physiol. 2012. PMID: 22338083 Free PMC article.
-
Transport of organic anions across the basolateral membrane of proximal tubule cells.Rev Physiol Biochem Pharmacol. 2003;146:95-158. doi: 10.1007/s10254-002-0003-8. Epub 2003 Jan 30. Rev Physiol Biochem Pharmacol. 2003. PMID: 12605306 Review.
Cited by
-
The additive effects of atorvastatin and insulin on renal function and renal organic anion transporter 3 function in diabetic rats.Sci Rep. 2017 Oct 19;7(1):13532. doi: 10.1038/s41598-017-13206-5. Sci Rep. 2017. PMID: 29051569 Free PMC article.
-
Phylogenetic, syntenic, and tissue expression analysis of slc22 genes in zebrafish (Danio rerio).BMC Genomics. 2016 Aug 12;17(1):626. doi: 10.1186/s12864-016-2981-y. BMC Genomics. 2016. PMID: 27519738 Free PMC article.
-
Physiology, structure, and regulation of the cloned organic anion transporters.Xenobiotica. 2008 Jul;38(7-8):889-935. doi: 10.1080/00498250801927435. Xenobiotica. 2008. PMID: 18668434 Free PMC article. Review.
-
Assessing the bioaccumulation potential of ionizable organic compounds: Current knowledge and research priorities.Environ Toxicol Chem. 2017 Apr;36(4):882-897. doi: 10.1002/etc.3680. Epub 2016 Dec 19. Environ Toxicol Chem. 2017. PMID: 27992066 Free PMC article. Review.
-
Impaired insulin signaling affects renal organic anion transporter 3 (Oat3) function in streptozotocin-induced diabetic rats.PLoS One. 2014 May 6;9(5):e96236. doi: 10.1371/journal.pone.0096236. eCollection 2014. PLoS One. 2014. Retraction in: PLoS One. 2018 Aug 21;13(8):e0202898. doi: 10.1371/journal.pone.0202898. PMID: 24801871 Free PMC article. Retracted.
References
-
- Bahn A, Knabe M, Hagos Y, Rödiger M, Godehardt S, Graber-Neufeld DS, Evans KK, Burckhardt G, Wright SH. Interaction of the metal chelator 2,3-dimercapto-1-propanesulfonate with the rabbit multispecific organic anion transporter 1 (rbOAT1) Mol Pharmacol. 2002;62:1128 –1136. - PubMed
-
- Bakhiya A, Bahn A, Burckhardt G, Wolff N. Human organic anion transporter 3 (hOAT3) can operate as an exchanger and mediate secretory urate flux. Cell Physiol Biochem. 2003;13:249 –256. - PubMed
-
- Brady KP, Dushkin H, Fornzler D, Koike T, Magner F, Her H, Gullans S, Segre GV, Green RM, Beier DR. A novel putative transporter maps to the osteosclerosis (oc) mutation and is not expressed in the oc mutant mouse. Genomics. 1999;56:254 –261. - PubMed
-
- Burckhardt BC, Brai S, Wallis S, Krick W, Wolff NA, Burckhardt G. Transport of cimetidine by flounder and human renal organic anion transporter 1. Am J Physiol Renal Physiol. 2003;284:F503–F509. - PubMed
-
- Burckhardt BC, Wolff NA, Burckhardt G. Electrophysiologic characterization of an organic anion transporter cloned from winter flounder kidney (fROAT) J Am Soc Nephrol. 2000;11:9 –17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

