Inhibition of cyclooxygenase isoforms in late- but not midgestation decreases contractility of the ductus arteriosus and prevents postnatal closure in mice
- PMID: 16857891
- PMCID: PMC2819844
- DOI: 10.1152/ajpregu.00259.2006
Inhibition of cyclooxygenase isoforms in late- but not midgestation decreases contractility of the ductus arteriosus and prevents postnatal closure in mice
Abstract
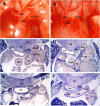
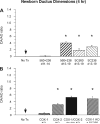
Use of cyclooxygenase (COX) inhibitors to delay preterm birth is complicated by in utero constriction of the ductus arteriosus and delayed postnatal closure. Delayed postnatal closure has been attributed to loss of vasa vasorum flow and ductus wall ischemia resulting from constriction in utero. We used the murine ductus (which does not depend on vasa vasorum flow) to determine whether delayed postnatal closure may be because of mechanisms independent of in utero constriction. Acute inhibition of both COX isoforms constricted the fetal ductus on days 18 and 19 (term) but not earlier in gestation; COX-2 inhibition constricted the fetal ductus more than COX-1 inhibition. In contrast, mice exposed to prolonged inhibition of COX-1, COX-2, or both COX isoforms (starting on day 15, when the ductus does not respond to the inhibitors) had no contractile response to the inhibitors on days 18 or 19. Newborn mice closed their ductus within 4 h of birth. Prolonged COX inhibition on days 11-14 of gestation had no effect on newborn ductal closure; however, prolonged COX inhibition on days 15-19 resulted in delayed ductus closure despite exposure to 80% oxygen after birth. Similarly, targeted deletion of COX-2 alone, or COX-1/COX-2 together, impaired postnatal ductus closure. Nitric oxide inhibition did not prevent the delay in ductus closure. These data show that impaired postnatal ductus closure is not the result of in utero ductus constriction or upregulation of nitric oxide synthesis. They are consistent with a novel role for prostaglandins in ductus arteriosus contractile development.
Figures

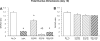
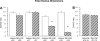

References
-
- Abe M, Hasegawa K, Wada H, Morimoto T, Yanazume T, Kawamura T, Hirai M, Furukawa Y, Kita T. GATA-6 is involved in PPAR-gamma-mediated activation of differentiated phenotype in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2003;23:404–410. - PubMed
-
- Badawi AF, El-Sohemy A, Stephen LL, Ghoshal AK, Archer MC. The effect of dietary n-3 and n-6 polyunsaturated fatty acids on the expression of cyclooxygenase 1 and 2 and levels of p21ras in rat mammary glands. Carcinogenesis. 1998;19:905–910. - PubMed
-
- Bergwerff M, DeRuiter MC, Poelmann RE, Gittenbergerde Groot AC. Onset of elastogenesis and downregulation of smooth muscle actin as distinguishing phenomena in artery differentiation in the chick embryo. Anat Embryol (Berl) 1996;194:545–557. - PubMed
-
- Brook MM, Heymann MA. Patent ductus arteriosus. In: Emmanouilides GC, Riemenscneider TA, Allen HD, Gutgesell HP, editors. Moss and Adams’ Heart Disease in Infants, Children, and Adolescents: Including the Fetus and Young Adult. 5th ed. Williams & Wilkins; Baltimore, MD: 1995. pp. 746–764.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

