Plasma inhibitory activity (PIA): a pharmacodynamic assay reveals insights into the basis for cytotoxic response to FLT3 inhibitors
- PMID: 16857987
- PMCID: PMC1895426
- DOI: 10.1182/blood-2006-04-015743
Plasma inhibitory activity (PIA): a pharmacodynamic assay reveals insights into the basis for cytotoxic response to FLT3 inhibitors
Abstract
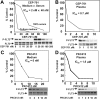
We have developed a useful surrogate assay for monitoring the efficacy of FLT3 inhibition in patients treated with oral FLT3 inhibitors. The plasma inhibitory activity (PIA) for FLT3 correlates with clinical activity in patients treated with CEP-701 and PKC412. Using the PIA assay, along with in vitro phosphorylation and cytotoxicity assays in leukemia cells, we compared PKC412 and its metabolite, CGP52421, with CEP-701. While both drugs could effectively inhibit FLT3 in vitro, CEP-701 was more cytotoxic to primary samples at comparable levels of FLT3 inhibition. PKC412 appears to be more selective than CEP-701 and therefore less effective at inducing cytotoxicity in primary acute myeloid leukemia (AML) samples in vitro. However, the PKC412 metabolite CGP52421 is less selective than its parent compound, PKC412, and is more cytotoxic against primary blast samples at comparable levels of FLT3 inhibition. The plasma inhibitory activity assay represents a useful correlative tool in the development of small-molecule inhibitors. Our application of this assay has revealed that the metabolite CGP52421 may contribute a significant portion of the antileukemia activity observed in patients receiving oral PKC412. Additionally, our results suggest that nonselectivity may constitute an important component of the cytotoxic effect of FLT3 inhibitors in FLT3-mutant AML.
Figures

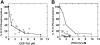
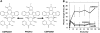

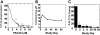

References
-
- Levis M, Small D. FLT3: ITDoes matter in leukemia. Leukemia. 2003;17: 1738-1752. - PubMed
-
- Levis M, Small D. FLT3 tyrosine kinase inhibitors. Int J Hematol. 2005;82: 100-107. - PubMed
-
- Smith BD, Levis M, Beran M, et al. Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood. 2004;103: 3669-3676. - PubMed
-
- Stone RM, DeAngelo DJ, Klimek V, et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood. 2005;105: 54-60. - PubMed
-
- Levis M, Smith BD, Beran M, et al. A randomized, open-label study of lestaurtinib (CEP-701), an oral FLT3 inhibitor, administered in sequence with chemotherapy in patients with relapsed AML harboring FLT3 activating mutations: clinical response correlates with successful FLT3 inhibition [abstract]. Blood. 2005;106: 121a. Abstract 403.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

