Gut-enriched Krüppel-like factor interaction with Smad3 inhibits myofibroblast differentiation
- PMID: 16858008
- PMCID: PMC1899300
- DOI: 10.1165/rcmb.2006-0043OC
Gut-enriched Krüppel-like factor interaction with Smad3 inhibits myofibroblast differentiation
Abstract
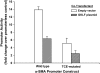
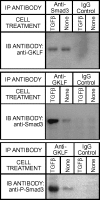
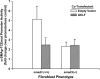
Gut-enriched Krüppel-like factor (GKLF) has been reported to partially inhibit alpha-smooth muscle actin (alpha-SMA) gene transcription by competing for binding to the TGF-beta control element (TCE) with known activators such as Sp1 and other Krüppel-like factors. This incomplete inhibition via the TCE suggests an additional mechanism, which was evaluated in this study. The results showed that an alpha-SMA promoter mutated in the TCE remained susceptible to inhibition by GKLF in rat lung fibroblasts consistent with the existence of an additional TCE-independent mechanism. Since TGF-beta- induced alpha-SMA expression is Smad3-dependent, potential interaction between GKLF and Smad3 was examined as a basis for this additional inhibitory mechanism. Co-immunoprecipitation and yeast two-hybrid assays revealed that GKLF could bind Smad3 through the Smad3 MH2 domain. Electrophoretic mobility shift assays and ChIP assay indicated that this GKLF-Smad3 interaction inhibited Smad3 binding to the Smad3-binding element (SBE) in the alpha-SMA promoter, and the activity of an SBE containing artificial promoter. Further analysis using smad3(-/-) fibroblasts confirmed that the TCE-independent inhibition by GKLF was dependent on Smad3. These data taken together suggest that in addition to inhibition via the TCE, GKLF represses alpha-SMA gene expression by interacting with Smad3 to prevent Smad3 binding to the SBE. It represents the first evidence to directly link GKLF with Smad3, a key intracellular mediator of TGF-beta signaling, which should lead to a clearer understanding of the mechanism of how GKLF regulates TGF-beta-induced gene expression.
Figures



Similar articles
-
A transforming growth factor-beta control element required for SM alpha-actin expression in vivo also partially mediates GKLF-dependent transcriptional repression.J Biol Chem. 2003 Nov 28;278(48):48004-11. doi: 10.1074/jbc.M301902200. Epub 2003 Sep 10. J Biol Chem. 2003. PMID: 12970361
-
Ligand-activated PPARδ upregulates α-smooth muscle actin expression in human dermal fibroblasts: A potential role for PPARδ in wound healing.J Dermatol Sci. 2015 Dec;80(3):186-95. doi: 10.1016/j.jdermsci.2015.10.005. Epub 2015 Oct 9. J Dermatol Sci. 2015. PMID: 26481780
-
Notch induces myofibroblast differentiation of alveolar epithelial cells via transforming growth factor-{beta}-Smad3 pathway.Am J Respir Cell Mol Biol. 2011 Jul;45(1):136-44. doi: 10.1165/rcmb.2010-0140oc. Am J Respir Cell Mol Biol. 2011. PMID: 21749980
-
Smad3 mediates transforming growth factor-beta-induced alpha-smooth muscle actin expression.Am J Respir Cell Mol Biol. 2003 Sep;29(3 Pt 1):397-404. doi: 10.1165/rcmb.2003-0063OC. Epub 2003 Apr 17. Am J Respir Cell Mol Biol. 2003. PMID: 12702545
-
[The mechanism of transforming growth factor beta1 in myofibroblast differentiation].Zhonghua Wai Ke Za Zhi. 2007 Jul 15;45(14):986-9. Zhonghua Wai Ke Za Zhi. 2007. PMID: 17961388 Chinese.
Cited by
-
Mesenchymal-specific deletion of C/EBPβ suppresses pulmonary fibrosis.Am J Pathol. 2012 Jun;180(6):2257-67. doi: 10.1016/j.ajpath.2012.02.010. Epub 2012 Apr 11. Am J Pathol. 2012. PMID: 22503555 Free PMC article.
-
The myofibroblast: one function, multiple origins.Am J Pathol. 2007 Jun;170(6):1807-16. doi: 10.2353/ajpath.2007.070112. Am J Pathol. 2007. PMID: 17525249 Free PMC article. Review.
-
Kruppel-like factor 4 inhibits epithelial-to-mesenchymal transition through regulation of E-cadherin gene expression.J Biol Chem. 2010 May 28;285(22):16854-63. doi: 10.1074/jbc.M110.114546. Epub 2010 Mar 31. J Biol Chem. 2010. PMID: 20356845 Free PMC article.
-
BMP signaling orchestrates a transcriptional network to control the fate of mesenchymal stem cells in mice.Development. 2017 Jul 15;144(14):2560-2569. doi: 10.1242/dev.150136. Epub 2017 Jun 2. Development. 2017. PMID: 28576771 Free PMC article.
-
Mechanisms of Ventilator-induced Lung Injury: Is the Elafin in the Room?Am J Respir Cell Mol Biol. 2018 Nov;59(5):531-532. doi: 10.1165/rcmb.2018-0205ED. Am J Respir Cell Mol Biol. 2018. PMID: 30095977 Free PMC article. No abstract available.
References
-
- Pardo A, Selman M. Idiopathic pulmonary fibrosis: new insights in its pathogenesis. Int J Biochem Cell Biol 2002;34:1534–1538. - PubMed
-
- Campbell JH, Kocher O, Skalli O, Gabbiani G, Campbell GR. Cytodifferentiation and expression of alpha-smooth muscle actin mRNA and protein during primary culture of aortic smooth muscle cells: correlation with cell density and proliferative state. Arteriosclerosis 1989;9: 633–643. - PubMed
-
- Woodcock-Mitchell J, Mitchell JJ, Low RB, Kieny M, Sengel P, Rubbia L, Skalli O, Jackson B, Gabbiani G. Alpha-smooth muscle actin is transiently expressed in embryonic rat cardiac and skeletal muscles. Differentiation 1988;39:161–166. - PubMed
-
- Hu B, Wu Z, Phan SH. Smad3 mediates transforming growth factor-β–induced alpha-smooth muscle actin expression. Am J Respir Cell Mol Biol 2003;29:397–404. - PubMed
-
- Darby I, Skalli O, Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest 1990;63:21–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

