The ILAILL study: iloprost as adjuvant to surgery for acute ischemia of lower limbs: a randomized, placebo-controlled, double-blind study by the italian society for vascular and endovascular surgery
- PMID: 16858180
- PMCID: PMC1602150
- DOI: 10.1097/01.sla.0000217555.49001.ca
The ILAILL study: iloprost as adjuvant to surgery for acute ischemia of lower limbs: a randomized, placebo-controlled, double-blind study by the italian society for vascular and endovascular surgery
Abstract
Summary background data: High rate of complications has been reported following revascularization for acute limb ischemia (ALI). No adjuvant pharmacologic treatment, apart from anticoagulation and standard perioperative care, has been shown clinically effective.
Objective: Aim of this study was to evaluate the effects of the prostacyclin analog iloprost as adjuvant to surgery for ALI.
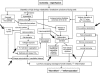
Methods: A total of 300 patients were randomly assigned to receive perioperative iloprost (intra-arterial, intraoperative bolus of 3000 ng, plus intravenous infusion of 0.5-2.0 ng/kg/min for 6 hours/day for 4-7 days following surgery), or placebo. The primary endpoint was the combined incidence of death and amputation at 3-month follow-up. Secondary endpoints were the incidence of each single major complication, total event rate, symptomatology, and tolerability.
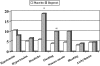
Results: The combined incidence of death and amputation was 19.9% in the placebo and 14.1% in the iloprost group (relative risk, 1.56; 95% confidence interval, 0.89-2.75, P = 0.12, Cox regression analysis). A statistically significant lower mortality (4.7%) was reported in patients receiving iloprost, compared with controls (10.6%; relative risk, 2.61; 95% confidence interval, 1.07-6.37, P = 0.03). The overall incidence of fatal plus major cardiovascular events was 33.1% and 22.8% in placebo and iloprost groups, respectively (relative risk, 1.61; 95% confidence interval, 1.04-2.49, P = 0.03). No serious adverse reactions occurred after iloprost administration, nor differences in the incidence of bleeding or hypotension between treatment groups.
Conclusions: Although at lower levels than previously reported, our results confirm the severity of ALI. Iloprost as adjuvant to surgery significantly reduced mortality and overall major event rate. Further data are needed to support this finding, and to face a still open medical issue.
Figures

References
-
- Management of Peripheral Arterial Disease (PAD). Trans-Atlantic Inter-Society Consensus (TASC). J Vasc Surg. 2000;31(suppl):1–296. - PubMed
-
- Ouriel K, Veith FJ, Sasahara AA, et al. A comparison of recombinant urokinase with vascular surgery as initial treatment for acute arterial occlusion of the legs. N Engl J Med. 1998;338:1105–1111. - PubMed
-
- Aune S, Trippestad A. Operative mortality and long-term survival of patients operated on for acute lower extremity ischemia. Eur J Vasc Endovasc Surg. 1998;15:143–146. - PubMed
-
- Braithwaite BD, Davies B, Birch PA, et al. Management of acute leg ischemia in the elderly. Br J Surg. 1998;85:217–220. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

