Glial inhibition of CNS axon regeneration
- PMID: 16858390
- PMCID: PMC2693386
- DOI: 10.1038/nrn1956
Glial inhibition of CNS axon regeneration
Abstract
Damage to the adult CNS often leads to persistent deficits due to the inability of mature axons to regenerate after injury. Mounting evidence suggests that the glial environment of the adult CNS, which includes inhibitory molecules in CNS myelin as well as proteoglycans associated with astroglial scarring, might present a major hurdle for successful axon regeneration. Here, we evaluate the molecular basis of these inhibitory influences and their contributions to the limitation of long-distance axon repair and other types of structural plasticity. Greater insight into glial inhibition is crucial for developing therapies to promote functional recovery after neural injury.
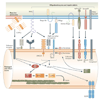
Figures


References
-
- Zito K, Svoboda K. Activity-dependent synaptogenesis in the adult mammalian cortex. Neuron. 2002;35:1015–1017. - PubMed
-
- Zuo Y, Yang G, Kwon E, Gan WB. Long-term sensory deprivation prevents dendritic spine loss in primary somatosensory cortex. Nature. 2005;436:261–265. - PubMed
-
- Edgerton VR, Tillakaratne NJ, Bigbee AJ, de Leon RD, Roy RR. Plasticity of the spinal neural circuitry after injury. Annu. Rev. Neurosci. 2004;27:145–167. - PubMed
-
- Ramón y Cajal S. Degeneration and Regeneration of the Nervous System. London: Oxford Univ. Press; 1928.
-
-
Tom VJ, Steinmetz MP, Miller JH, Doller CM, Silver J. Studies on the development and behavior of the dystrophic growth cone, the hallmark of regeneration failure, in an in vitro model of the glial scar and after spinal cord injury. J. Neurosci. 2004;24:6531–6539. An interesting study analysing the morphological changes that constitute the dystrophic growth cones of lesioned axons in the hostile CNS environment.
-
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

