An essential complementary role of NF-kappaB pathway to microbicidal oxidants in Drosophila gut immunity
- PMID: 16858400
- PMCID: PMC1538556
- DOI: 10.1038/sj.emboj.7601233
An essential complementary role of NF-kappaB pathway to microbicidal oxidants in Drosophila gut immunity
Abstract
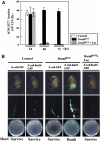
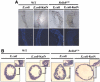
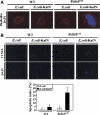
In the Drosophila gut, reactive oxygen species (ROS)-dependent immunity is critical to host survival. This is in contrast to the NF-kappaB pathway whose physiological function in the microbe-laden epithelia has yet to be convincingly demonstrated despite playing a critical role during systemic infections. We used a novel in vivo approach to reveal the physiological role of gut NF-kappaB/antimicrobial peptide (AMP) system, which has been 'masked' in the presence of the dominant intestinal ROS-dependent immunity. When fed with ROS-resistant microbes, NF-kappaB pathway mutant flies, but not wild-type flies, become highly susceptible to gut infection. This high lethality can be significantly reduced by either re-introducing Relish expression to Relish mutants or by constitutively expressing a single AMP to the NF-kappaB pathway mutants in the intestine. These results imply that the local 'NF-kappaB/AMP' system acts as an essential 'fail-safe' system, complementary to the ROS-dependent gut immunity, during gut infection with ROS-resistant pathogens. This system provides the Drosophila gut immunity the versatility necessary to manage sporadic invasion of virulent pathogens that somehow counteract or evade the ROS-dependent immunity.
Figures




References
-
- Bader MW, Sanowar S, Daley ME, Schneider AR, Cho U, Xu W, Klevit RE, Le Moual H, Miller SI (2005) Recognition of antimicrobial peptides by a bacterial sensor kinase. Cell 122: 461–472 - PubMed
-
- Bevins CL (2004) The Paneth cell and the innate immune response. Curr Opin Gastroenterol 20: 572–580 - PubMed
-
- Boutros M, Agaisse H, Perrimon N (2002) Sequential activation of signaling pathways during innate immune responses in Drosophila. Dev Cell 3: 711–722 - PubMed
-
- Brand AH, Perrimon N (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

