The inner nuclear membrane protein emerin regulates beta-catenin activity by restricting its accumulation in the nucleus
- PMID: 16858403
- PMCID: PMC1523183
- DOI: 10.1038/sj.emboj.7601230
The inner nuclear membrane protein emerin regulates beta-catenin activity by restricting its accumulation in the nucleus
Abstract
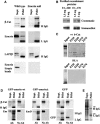
Emerin is a type II inner nuclear membrane (INM) protein of unknown function. Emerin function is likely to be important because, when it is mutated, emerin promotes both skeletal muscle and heart defects. Here we show that one function of Emerin is to regulate the flux of beta-catenin, an important transcription coactivator, into the nucleus. Emerin interacts with beta-catenin through a conserved adenomatous polyposis coli (APC)-like domain. When GFP-emerin is expressed in HEK293 cells, beta-catenin is restricted to the cytoplasm and beta-catenin activity is inhibited. In contrast, expression of an emerin mutant, lacking its APC-like domain (GFP-emerinDelta), dominantly stimulates beta-catenin activity and increases nuclear accumulation of beta-catenin. Human fibroblasts that are null for emerin have an autostimulatory growth phenotype. This unusual growth phenotype arises through enhanced nuclear accumulation and activity of beta-catenin and can be replicated in wild-type fibroblasts by transfection with constitutively active beta-catenin. Our results support recent findings that suggest that INM proteins can influence signalling pathways by restricting access of transcription coactivators to the nucleus.
Figures






References
-
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W (1996) Functional interaction of beta-catenin with the transcription factor LEF-1. Nature 382: 638–642 - PubMed
-
- Bione S, Maestrini E, Rivella S, Mancini M, Regis S, Romeo G, Toniolo D. (1994) Identification of a novel X-linked gene responsible for Emery–Dreifuss muscular dystrophy. Nat Genet 8: 323–327 - PubMed
-
- Bonne G, Di Barletta MR, Varnous S, Becane HM, Hammouda EH, Merlini L, Muntoni F, Greenberg CR, Gary F, Urtizberea JA, Duboc D, Fardeau M, Toniolo D, Schwartz K (1999) Mutations in the gene encoding lamin A/C cause autosomal dominant Emery–Dreifuss muscular dystrophy. Nat Genet 21: 285–288 - PubMed
-
- Brill LM, Salomon AR, Ficarro SB, Mukherjic M, Stettler-Gill M, Peters EC (2004) Robust phosphoproteomic profiling of tyrosine phosphorylation sites from human T cells using immobilised metal affinity chromatography and tandem mass spectrometry. Anal Chem 76: 2763–2772 - PubMed
-
- Cartegni L, di Barletta MR, Barresi R, Squarzoni S, Sabatelli P, Maraldi N, Mora M, Di Blasi C, Cornelio F, Merlini L, Villa A, Cobianchi F, Toniolo D (1997) Heart-specific localization of emerin: new insights into Emery–Dreifuss muscular dystrophy. Hum Mol Genet 13: 2257–2264 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

