A structural analysis of asymmetry required for catalytic activity of an ABC-ATPase domain dimer
- PMID: 16858415
- PMCID: PMC1523178
- DOI: 10.1038/sj.emboj.7601208
A structural analysis of asymmetry required for catalytic activity of an ABC-ATPase domain dimer
Abstract
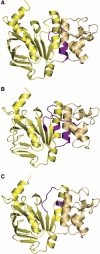
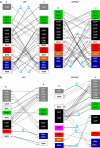
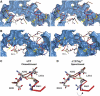
The ATP-binding cassette (ABC)-transporter haemolysin (Hly)B, a central element of a Type I secretion machinery, acts in concert with two additional proteins in Escherichia coli to translocate the toxin HlyA directly from the cytoplasm to the exterior. The basic set of crystal structures necessary to describe the catalytic cycle of the isolated HlyB-NBD (nucleotide-binding domain) has now been completed. This allowed a detailed analysis with respect to hinge regions, functionally important key residues and potential energy storage devices that revealed many novel features. These include a structural asymmetry within the ATP dimer that was significantly enhanced in the presence of Mg2+, indicating a possible functional asymmetry in the form of one open and one closed phosphate exit tunnel. Guided by the structural analysis, we identified two amino acids, closing one tunnel by an apparent salt bridge. Mutation of these residues abolished ATP-dependent cooperativity of the NBDs. The implications of these new findings for the coupling of ATP binding and hydrolysis to functional activity are discussed.
Figures


References
-
- Chang G (2003) Structure of MsbA from Vibrio cholera: a multidrug resistance ABC transporter homolog in a closed conformation. J Mol Biol 330: 419–430 - PubMed
-
- Chang G, Roth CB (2001) Structure of MsbA from E. coli: a homolog of the multidrug resistance ATP binding cassette (ABC) transporters. Science 293: 1793–1800 - PubMed
-
- Chen J, Lu G, Lin J, Davidson AL, Quiocho FA (2003) A tweezers-like motion of the ATP-binding cassette dimer in an ABC transport cycle. Mol Cell 12: 651–661 - PubMed
-
- Davidson AL, Chen J (2004) ATP-binding cassette transporters in bacteria. Annu Rev Biochem 73: 241–268 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources

