Clinical recommendations for the use of recombinant human erythropoietin in patients with hepatitis C virus being treated with ribavirin
- PMID: 16858501
- PMCID: PMC2659916
- DOI: 10.1155/2006/497059
Clinical recommendations for the use of recombinant human erythropoietin in patients with hepatitis C virus being treated with ribavirin
Abstract
Today, combination antiviral therapy with pegylated interferon-alpha and ribavirin (RBV) allows many patients infected with hepatitis C virus (HCV) to achieve a sustained virological response, which is equivalent to cure. Data also support the clinical benefit of combination antiviral therapy in patients coinfected with HCV and HIV, and in patients who have received a liver transplant. Antiviral therapy with pegylated interferon-alpha and RBV is, however, associated with a high incidence and significant magnitude of anemia. This anemia may have several mechanisms, including bone marrow suppression and hemolysis. In addition, patients coinfected with HIV may have both pre-existing and RBV-associated anemia. Management of anemia in patients with HCV through RBV dose reduction or treatment discontinuation may compromise the effectiveness of treatment, because studies have demonstrated that treatment adherence or maintenance of antiviral therapy dose is an important predictor of sustained virological response. Anemia associated with combination antiviral therapy in patients with HCV is frequently associated with an inadequate or blunted endogenous erythropoietin response. Accumulating evidence now supports the use of recombinant human erythropoietin (rHuEpo) to manage anemia in these patients, with the objective of maintaining the RBV dose, but clinical standards are lacking. The present article reviews the data relevant to the use of rHuEpo in this patient population and proposes a set of clinical practice standards to assist clinicians in selecting patients for rHuEpo and in implementing rHuEpo therapy effectively.
De nos jours, la polythérapie antivirale à l’interféron pégylé alpha et à la ribavirine (RBV) permet à de nombreux patients infectés par le virus de l’hépatite C (VHC) de profiter d’une réaction virologique soutenue, équivalant à la guérison. Les données appuient également le bienfait clinique de la polythérapie antivirale chez les patients co-infectés par le VHC et le VIH et chez les patients greffés du foie.
La thérapie antivirale à l’interféron pégylé alpha et à la RBV s’associe toutefois à une forte incidence d’anémie de grande envergure. Cette anémie peut se manifester par divers mécanismes, y compris la suppression médullaire et l’hémolyse. De plus, les patients co-infectés par le VIH peuvent souffrir à la fois d’anémie préexistante et d’anémie causée par la RBV. La prise en charge de l’anémie chez les patients atteints du VHC par une réduction de la dose de RBV ou l’abandon du traitement peut compromettre l’efficacité du traitement. En effet, les études ont démontré que l’adhésion au traitement ou le maintien de la dose antivirale constituent des prédicteurs importants d’une réaction virologique soutenue.
L’anémie associée à la polythérapie antivirale chez les patients atteints du VHC s’associe souvent à l’insuffisance ou à l’émoussement de la réaction de l’érythropoiétine endogène. Les données probantes s’accumulent pour soutenir le recours à l’érythropoiétine humaine recombinante (rHuEpo) afin de prendre en charge l’anémie chez ces patients en vue de maintenir la dose de RBV, mais il n’existe pas de normes cliniques à cet effet. Le présent article analyse les données reliées à l’utilisation de rHuEpo au sein de cette population de patients et inclut une série de normes de pratique clinique pour aider les cliniciens à sélectionner les patients qui prendront de la rHuEpo et à implanter une thérapie efficace à la rHuEpo.
Figures

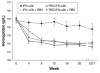

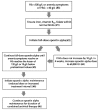
Comment in
-
Erythropoietin and hepatitis C therapy: useful adjuvant therapy but remember to treat the patient and not just a number.Can J Gastroenterol. 2006 Aug;20(8):519-20. doi: 10.1155/2006/793589. Can J Gastroenterol. 2006. PMID: 16967572 Free PMC article. No abstract available.
Similar articles
-
Epoetin alfa maintains ribavirin dose in HCV-infected patients: a prospective, double-blind, randomized controlled study.Gastroenterology. 2004 May;126(5):1302-11. doi: 10.1053/j.gastro.2004.01.027. Gastroenterology. 2004. PMID: 15131791 Clinical Trial.
-
Role of epoetin alfa in maintaining ribavirin dose.Gastroenterol Clin North Am. 2004 Mar;33(1 Suppl):S25-35. doi: 10.1016/j.gtc.2003.12.002. Gastroenterol Clin North Am. 2004. PMID: 15081101
-
Treatment of hepatitis C and anemia in human immunodeficiency virus-infected patients.J Infect Dis. 2002 May 15;185 Suppl 2:S128-37. doi: 10.1086/340199. J Infect Dis. 2002. PMID: 12001034 Review.
-
Comparison of high ribavirin induction versus standard ribavirin dosing, plus peginterferon-α for the treatment of chronic hepatitis C in HIV-infected patients: the PERICO trial.J Infect Dis. 2012 Sep 15;206(6):961-8. doi: 10.1093/infdis/jis449. Epub 2012 Jul 17. J Infect Dis. 2012. PMID: 22807523 Clinical Trial.
-
Epoetin alfa treatment for acute anaemia during interferon plus ribavirin combination therapy for chronic hepatitis C.J Viral Hepat. 2004 May;11(3):191-7. doi: 10.1111/j.1365-2893.2004.00506.x. J Viral Hepat. 2004. PMID: 15117320 Review.
Cited by
-
Use of agents stimulating erythropoiesis in digestive diseases.World J Gastroenterol. 2009 Oct 7;15(37):4675-85. doi: 10.3748/wjg.15.4675. World J Gastroenterol. 2009. PMID: 19787831 Free PMC article. Review.
-
Hepatitis C virus reinfection after liver transplant: New chances and new challenges in the era of direct-acting antiviral agents.World J Hepatol. 2015 Mar 27;7(3):532-8. doi: 10.4254/wjh.v7.i3.532. World J Hepatol. 2015. PMID: 25848476 Free PMC article. Review.
-
IMPACT OF THE PEGYLATED-INTERFERON AND RIBAVIRIN THERAPY ON THE TREATMENT-RELATED MORTALITY OF PATIENTS WITH CIRRHOSIS DUE TO HEPATITIS C VIRUS.Rev Inst Med Trop Sao Paulo. 2016;58:37. doi: 10.1590/S1678-9946201658037. Epub 2016 May 24. Rev Inst Med Trop Sao Paulo. 2016. PMID: 27253739 Free PMC article.
-
Erythropoietin and hepatitis C therapy: useful adjuvant therapy but remember to treat the patient and not just a number.Can J Gastroenterol. 2006 Aug;20(8):519-20. doi: 10.1155/2006/793589. Can J Gastroenterol. 2006. PMID: 16967572 Free PMC article. No abstract available.
-
Use of hematopoietic growth factor in the management of hematological side effects associated to antiviral treatment for HCV hepatitis.Mediterr J Hematol Infect Dis. 2010 Mar 31;2(1):e2010003. doi: 10.4084/MJHID.2010.003. Mediterr J Hematol Infect Dis. 2010. PMID: 21415945 Free PMC article.
References
-
- Sherman M, Bain V, Villeneuve JP, et al. Management of Viral Hepatitis: A Canadian Consensus Conference 2004. The Public Health Agency of Canada<http://www.phac-aspc.gc.ca> (Version current at September 21, 2005).
-
- Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: A randomised trial. Lancet. 2001;358:958–65. - PubMed
-
- Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–82. - PubMed
-
- Ferenci P, Fried MW, Shiffman ML, et al. Predicting sustained virological responses in chronic hepatitis C patients treated with peginterferon alfa-2a (40 KD)/ribavirin. J Hepatol. 2005;43:425–33. - PubMed
-
- Davis GL, Wong JB, McHutchison JG, Manns MP, Harvey J, Albrecht J. Early virologic response to treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C. Hepatology. 2003;38:645–52. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
