Rapid stimulation of tyrosine phosphorylation signals downstream of G-protein-coupled receptors for thromboxane A2 in human platelets
- PMID: 16859489
- PMCID: PMC1635449
- DOI: 10.1042/BJ20061015
Rapid stimulation of tyrosine phosphorylation signals downstream of G-protein-coupled receptors for thromboxane A2 in human platelets
Abstract
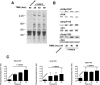
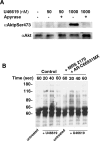
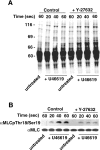
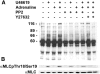
Signals ensuing from trimeric G-protein-coupled receptors synergize to induce platelet activation. At low doses, the thromboxane A2 analogue U46619 does not activate integrin alphaIIbbeta3 or trigger platelet aggregation, but it induces shape changes. In the present study, we addressed whether low doses of U46619 trigger tyrosine phosphorylation independently of integrin alphaIIbbeta3 activation and ADP secretion, and synergize with adrenaline (epinephrine) to induce aggregation in acetylsalicylic acid (aspirin)-treated platelets. Low doses of U46619 triggered tyrosine phosphorylation of different proteins, including FAK (focal adhesion kinase), Src and Syk, independently of signals ensuing from integrin alphaIIbbeta3 or ADP receptors engaged by secreted ADP. The G(12/13)-mediated Rho/Rho-kinase pathway was also increased by low doses of U46619; however, this pathway was not upstream of tyrosine phosphorylation, because this occurred in the presence of the Rho-kinase inhibitor Y-27632. Although low doses of U46619 or adrenaline alone were unable to trigger platelet aggregation and integrin alphaIIbbeta3 activation, the combination of the two stimuli effectively induced these responses. PP2, a tyrosine kinase inhibitor, and Y-27632 inhibited platelet activation induced by low doses of U46619 plus adrenaline and, when used in combination, totally suppressed this platelet response. In addition, the two inhibitors selectively blocked tyrosine kinases and the Rho/Rho-kinase pathway respectively. These findings suggest that both tyrosine phosphorylation and the Rho/Rho-kinase pathway are required to activate platelet aggregation via G(12/13) plus G(z) signalling.
Figures

Similar articles
-
A new role for FcgammaRIIA in the potentiation of human platelet activation induced by weak stimulation.Cell Signal. 2006 Jun;18(6):861-70. doi: 10.1016/j.cellsig.2005.07.014. Epub 2005 Oct 5. Cell Signal. 2006. PMID: 16169188
-
Secreted ADP plays a central role in thrombin-induced phospholipase D activation in human platelets.Thromb Haemost. 1998 Dec;80(6):976-81. Thromb Haemost. 1998. PMID: 9869170
-
Lysophosphatidic acid stimulation of platelets rapidly induces Ca2+-dependent dephosphorylation of cofilin that is independent of dense granule secretion and aggregation.Blood Cells Mol Dis. 2007 May-Jun;38(3):269-79. doi: 10.1016/j.bcmd.2007.01.002. Epub 2007 Feb 26. Blood Cells Mol Dis. 2007. PMID: 17321765
-
Gastrointestinal peptide signalling in health and disease.Eur J Surg Suppl. 2002;(587):23-38. Eur J Surg Suppl. 2002. PMID: 16144198 Review.
-
G-protein-coupled receptors as signaling targets for antiplatelet therapy.Arterioscler Thromb Vasc Biol. 2009 Apr;29(4):449-57. doi: 10.1161/ATVBAHA.108.176388. Epub 2008 Nov 20. Arterioscler Thromb Vasc Biol. 2009. PMID: 19023091 Review.
Cited by
-
Risk management profile of etoricoxib: an example of personalized medicine.Ther Clin Risk Manag. 2008 Oct;4(5):983-97. doi: 10.2147/tcrm.s3209. Ther Clin Risk Manag. 2008. PMID: 19209280 Free PMC article.
-
Aldosterone stimulates elastogenesis in cardiac fibroblasts via mineralocorticoid receptor-independent action involving the consecutive activation of Galpha13, c-Src, the insulin-like growth factor-I receptor, and phosphatidylinositol 3-kinase/Akt.J Biol Chem. 2009 Jun 12;284(24):16633-16647. doi: 10.1074/jbc.M109.008748. Epub 2009 Apr 16. J Biol Chem. 2009. PMID: 19372600 Free PMC article.
-
Antiplatelet Agents Inhibit the Generation of Platelet-Derived Microparticles.Front Pharmacol. 2016 Sep 16;7:314. doi: 10.3389/fphar.2016.00314. eCollection 2016. Front Pharmacol. 2016. PMID: 27695417 Free PMC article.
-
Interaction between src family kinases and rho-kinase in agonist-induced Ca2+-sensitization of rat pulmonary artery.Cardiovasc Res. 2008 Feb 1;77(3):570-9. doi: 10.1093/cvr/cvm073. Epub 2007 Nov 21. Cardiovasc Res. 2008. PMID: 18032393 Free PMC article.
-
Calcium-Dependent Src Phosphorylation and Reactive Oxygen Species Generation Are Implicated in the Activation of Human Platelet Induced by Thromboxane A2 Analogs.Front Pharmacol. 2018 Sep 26;9:1081. doi: 10.3389/fphar.2018.01081. eCollection 2018. Front Pharmacol. 2018. PMID: 30319416 Free PMC article.
References
-
- Jackson S. P., Schoenwaelder S. M. Antiplatelet therapy: in search of the ‘magic bullet’. Nat. Rev. Drug Discov. 2003;2:775–789. - PubMed
-
- Nieswandt B., Schulte V., Zywietz A., Gratacap M. P., Offermanns S. Costimulation of Gi- and G12/G13-mediated signaling pathways induces integrin αIIbβ3 activation in platelets. J. Biol. Chem. 2002;277:39493–39498. - PubMed
-
- Dorsam R. T., Kim S., Jin J., Kunapuli S. P. Coordinated signaling through both G12/13 and Gi pathways is sufficient to activate GPIIb/IIIa in human platelets. J. Biol. Chem. 2002;277:47588–47595. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

