Metabolic engineering of microbes for oligosaccharide and polysaccharide synthesis
- PMID: 16859553
- PMCID: PMC1544344
- DOI: 10.1186/1475-2859-5-25
Metabolic engineering of microbes for oligosaccharide and polysaccharide synthesis
Abstract
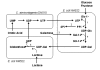
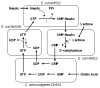
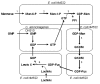
Metabolic engineering has recently been embraced as an effective tool for developing whole-cell biocatalysts for oligosaccharide and polysaccharide synthesis. Microbial catalysts now provide a practical means to derive many valuable oligosaccharides, previously inaccessible through other methods, in sufficient quantities to support research and clinical applications. The synthesis process based upon these microbes is scalable as it avoids expensive starting materials. Most impressive is the high product concentrations (up to 188 g/L) achieved through microbe-catalyzed synthesis. The overall cost for selected molecules has been brought to a reasonable range (estimated $30-50/g). Microbial synthesis of oligosaccharides and polysaccharides is a carbon-intensive and energy-intensive process, presenting some unique challenges in metabolic engineering. Unlike nicotinamide cofactors, the required sugar nucleotides are products of multiple interacting pathways, adding significant complexity to the metabolic engineering effort. Besides the challenge of providing the necessary mammalian-originated glycosyltransferases in active form, an adequate uptake of sugar acceptors can be an issue when another sugar is necessary as a carbon and energy source. These challenges are analyzed, and various strategies used to overcome these difficulties are reviewed in this article. Despite the impressive success of the microbial coupling strategy, there is a need to develop a single strain that can achieve at least the same efficiency. Host selection and the manner with which the synthesis interacts with the central metabolism are two important factors in the design of microbial catalysts. Additionally, unlike in vitro enzymatic synthesis, product degradation and byproduct formation are challenges of whole-cell systems that require additional engineering. A systematic approach that accounts for various and often conflicting requirements of the synthesis holds the key to deriving an efficient catalyst. Metabolic engineering strategies applied to selected polysaccharides (hyaluronan, alginate, and exopolysaccharides for food use) are reviewed in this article to highlight the recent progress in this area and similarity to challenges in oligosaccharide synthesis. Many naturally occurring microbes possess highly efficient mechanisms for polysaccharide synthesis. These mechanisms could potentially be engineered into a microbe for oligosaccharide and polysaccharide synthesis with enhanced efficiency.
Figures





Similar articles
-
Metabolic engineering for amino-, oligo-, and polysugar production in microbes.Appl Microbiol Biotechnol. 2016 Mar;100(6):2523-33. doi: 10.1007/s00253-015-7215-8. Epub 2016 Jan 19. Appl Microbiol Biotechnol. 2016. PMID: 26782743 Review.
-
Enzyme and microbial technology for synthesis of bioactive oligosaccharides: an update.Appl Microbiol Biotechnol. 2018 Apr;102(7):3017-3026. doi: 10.1007/s00253-018-8839-2. Epub 2018 Feb 23. Appl Microbiol Biotechnol. 2018. PMID: 29476402 Review.
-
The sweet branch of metabolic engineering: cherry-picking the low-hanging sugary fruits.Microb Cell Fact. 2015 Dec 9;14:197. doi: 10.1186/s12934-015-0389-z. Microb Cell Fact. 2015. PMID: 26655367 Free PMC article. Review.
-
Bacterial glycobiotechnology: A biosynthetic route for the production of biopharmaceutical glycans.Biotechnol Adv. 2023 Oct;67:108180. doi: 10.1016/j.biotechadv.2023.108180. Epub 2023 May 24. Biotechnol Adv. 2023. PMID: 37236328 Review.
-
Citrate stimulates oligosaccharide synthesis in metabolically engineered Agrobacterium sp.Appl Biochem Biotechnol. 2011 Jul;164(6):851-66. doi: 10.1007/s12010-011-9179-1. Epub 2011 Feb 8. Appl Biochem Biotechnol. 2011. PMID: 21302148
Cited by
-
Whole cell biosynthesis of a functional oligosaccharide, 2'-fucosyllactose, using engineered Escherichia coli.Microb Cell Fact. 2012 Apr 30;11:48. doi: 10.1186/1475-2859-11-48. Microb Cell Fact. 2012. PMID: 22545760 Free PMC article.
-
Metabolic engineering of lactic acid bacteria for the production of industrially important compounds.Comput Struct Biotechnol J. 2012 Oct 29;3:e201210003. doi: 10.5936/csbj.201210003. eCollection 2012. Comput Struct Biotechnol J. 2012. PMID: 24688663 Free PMC article. Review.
-
Microbial production of astilbin, a bioactive rhamnosylated flavanonol, from taxifolin.World J Microbiol Biotechnol. 2017 Feb;33(2):36. doi: 10.1007/s11274-017-2208-7. Epub 2017 Jan 24. World J Microbiol Biotechnol. 2017. PMID: 28120309
-
Transcriptomic Insights Into the Growth Phase- and Sugar-Associated Changes in the Exopolysaccharide Production of a High EPS-Producing Streptococcus thermophilus ASCC 1275.Front Microbiol. 2018 Aug 20;9:1919. doi: 10.3389/fmicb.2018.01919. eCollection 2018. Front Microbiol. 2018. PMID: 30177921 Free PMC article.
-
Systems biology and biological systems diversity for the engineering of microbial cell factories.Microb Cell Fact. 2007 Nov 20;6:35. doi: 10.1186/1475-2859-6-35. Microb Cell Fact. 2007. PMID: 18028536 Free PMC article. No abstract available.
References
-
- Wong C-H, Whitesides GM. Enzymes in Synthetic Organic Chemistry. 1. Vol. 12. New York: Oxford;
LinkOut - more resources
Full Text Sources
Other Literature Sources

