Modulation of anisotropy in electrospun tissue-engineering scaffolds: Analysis of fiber alignment by the fast Fourier transform
- PMID: 16859744
- PMCID: PMC2929953
- DOI: 10.1016/j.biomaterials.2006.06.014
Modulation of anisotropy in electrospun tissue-engineering scaffolds: Analysis of fiber alignment by the fast Fourier transform
Abstract
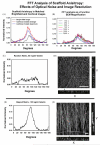
We describe the use of the fast Fourier transform (FFT) in the measurement of anisotropy in electrospun scaffolds of gelatin as a function of the starting conditions. In electrospinning, fiber alignment and overall scaffold anisotropy can be manipulated by controlling the motion of the collecting mandrel with respect to the source electrospinning solution. By using FFT to assign relative alignment values to an electrospun matrix it is possible to systematically evaluate how different processing variables impact the structure and material properties of a scaffold. Gelatin was suspended at varying concentrations (80, 100, 130, 150 mg/ml) and electrospun from 2,2,2 trifluoroethanol onto rotating mandrels (200-7000 RPM). At each starting concentration, fiber diameter remained constant over a wide range of mandrel RPM. Scaffold anisotropy developed as a function of fiber diameter and mandrel RPM. The induction of varying degrees of anisotropy imparted distinctive material properties to the electrospun scaffolds. The FFT is a rapid method for evaluating fiber alignment in tissue-engineering materials.
Figures




Similar articles
-
Incremental changes in anisotropy induce incremental changes in the material properties of electrospun scaffolds.Acta Biomater. 2007 Sep;3(5):651-61. doi: 10.1016/j.actbio.2007.02.010. Epub 2007 May 21. Acta Biomater. 2007. PMID: 17513181 Free PMC article.
-
Measuring fiber alignment in electrospun scaffolds: a user's guide to the 2D fast Fourier transform approach.J Biomater Sci Polym Ed. 2008;19(5):603-21. doi: 10.1163/156856208784089643. J Biomater Sci Polym Ed. 2008. PMID: 18419940 Review.
-
Image-based quantification of fiber alignment within electrospun tissue engineering scaffolds is related to mechanical anisotropy.J Biomed Mater Res A. 2016 Jul;104(7):1680-6. doi: 10.1002/jbm.a.35697. Epub 2016 Mar 17. J Biomed Mater Res A. 2016. PMID: 26939754
-
Regulation of material properties in electrospun scaffolds: Role of cross-linking and fiber tertiary structure.Acta Biomater. 2009 Jan;5(1):518-29. doi: 10.1016/j.actbio.2008.06.016. Epub 2008 Jul 4. Acta Biomater. 2009. PMID: 18676212 Free PMC article.
-
Gelatin-based electrospun and lyophilized scaffolds with nano scale feature for bone tissue engineering application: review.J Biomater Sci Polym Ed. 2022 Sep;33(13):1704-1758. doi: 10.1080/09205063.2022.2068943. Epub 2022 May 28. J Biomater Sci Polym Ed. 2022. PMID: 35443894 Review.
Cited by
-
Tissue Engineered Neural Constructs Composed of Neural Precursor Cells, Recombinant Spidroin and PRP for Neural Tissue Regeneration.Sci Rep. 2019 Feb 28;9(1):3161. doi: 10.1038/s41598-019-39341-9. Sci Rep. 2019. PMID: 30816182 Free PMC article.
-
Fabrication and Characterization of Aligned Flexible Lead-Free Piezoelectric Nanofibers for Wearable Device Applications.Nanomaterials (Basel). 2018 Mar 29;8(4):206. doi: 10.3390/nano8040206. Nanomaterials (Basel). 2018. PMID: 29596372 Free PMC article.
-
Oriented matrix promotes directional tubulogenesis.Acta Biomater. 2015 Jan;11:264-73. doi: 10.1016/j.actbio.2014.08.037. Epub 2014 Sep 8. Acta Biomater. 2015. PMID: 25219769 Free PMC article.
-
Cancer-associated fibroblasts promote directional cancer cell migration by aligning fibronectin.J Cell Biol. 2017 Nov 6;216(11):3799-3816. doi: 10.1083/jcb.201704053. Epub 2017 Oct 11. J Cell Biol. 2017. PMID: 29021221 Free PMC article.
-
Electrospun nanofiber scaffolds with gradations in fiber organization.J Vis Exp. 2015 Apr 19;(98):52626. doi: 10.3791/52626. J Vis Exp. 2015. PMID: 25938562 Free PMC article.
References
-
- Simpson DG. Dermal templates: the promise of true tissue regeneration. Exp Rev Med Dev. 2006 in press. - PubMed
-
- Telemeco TA, Ayres CE, Bowlin GL, Wnek GE, Boland ED, Cohen NM, et al. Regulation of cellular infiltration into tissue engineering scaffolds composed of submicron diameter fibers produced by electrospinning. Acta Biomater. 2005;1:377–85. - PubMed
-
- Zhang YZ, Venugopal J, Huang ZM, Lim CT, Ramakrishna S. Characterization of the surface biocompatibility of the electrospun PCL-collagen nanofibers using fibroblasts. Biomacromolecules. 2005;6(5):2583–9. - PubMed
-
- Boland ED, Telemeco TA, Simpson DG, Wnek GE, Bowlin GL. Utilizing acid pretreatment and electrospinning to improve biocompatibility of poly(glycolic acid) for tissue engineering. J Biomed Mater Res B Appl Biomater. 2004;71(1):144–52. - PubMed
-
- Kim K, Yu M, Zong X, Chiu J, Fang D, Seo YS, et al. Control of degradation rate and hydrophilicity in electrospun non-woven poly(d,l-lactide) nanofiber scaffolds for biomedical applications. Biomaterials. 2003;24(27):4977–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

