Prevention of postoperative nausea and vomiting by metoclopramide combined with dexamethasone: randomised double blind multicentre trial
- PMID: 16861255
- PMCID: PMC1539036
- DOI: 10.1136/bmj.38903.419549.80
Prevention of postoperative nausea and vomiting by metoclopramide combined with dexamethasone: randomised double blind multicentre trial
Abstract
Objectives: To determine whether 10 mg, 25 mg, or 50 mg metoclopramide combined with 8 mg dexamethasone, given intraoperatively, is more effective in preventing postoperative nausea and vomiting than 8 mg dexamethasone alone, and to assess benefit in relation to adverse drug reactions.
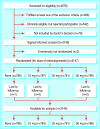
Design: Four-armed, parallel group, double blind, randomised controlled clinical trial.
Setting: Four clinics of a university hospital and four district hospitals in Germany.
Participants: 3140 patients who received balanced or regional anaesthesia during surgery.
Main outcome measures: Postoperative nausea and vomiting within 24 hours of surgery (primary end point); occurrence of adverse reactions.
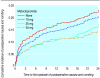
Results: Cumulative incidences (95% confidence intervals) of postoperative nausea and vomiting were 23.1% (20.2% to 26.0%), 20.6% (17.8% to 23.4%), 17.2% (14.6% to 19.8%), and 14.5% (12.0% to 17.0%) for 0 mg, 10 mg, 25 mg, and 50 mg metoclopramide. In the secondary analysis, 25 mg and 50 mg metoclopramide were equally effective at preventing early nausea (0-12 hours), but only 50 mg reduced late nausea and vomiting (> 12 hours). The most frequent adverse drug reactions were hypotension and tachycardia, with cumulative incidences of 8.8% (6.8% to 10.8%), 11.2% (9.0% to 13.4%), 12.9% (10.5% to 15.3%), and 17.9% (15.2% to 20.6%) for 0 mg, 10 mg, 25 mg, and 50 mg metoclopramide.
Conclusion: The addition of 50 mg metoclopramide to 8 mg dexamethasone (given intraoperatively) is an effective, safe, and cheap way to prevent postoperative nausea and vomiting. A reduced dose of 25 mg metoclopramide intraoperatively, with additional postoperative prophylaxis in high risk patients, may be equally effective and cause fewer adverse drug reactions.
Trial registration: Current Controlled Trials ISRCTN31625370 [controlled-trials.com].
Figures
Comment in
-
Postoperative nausea and vomiting.BMJ. 2006 Aug 12;333(7563):313-4. doi: 10.1136/bmj.333.7563.313. BMJ. 2006. PMID: 16902202 Free PMC article. No abstract available.
-
Preventing postoperative nausea and vomiting: prevention in context.BMJ. 2006 Aug 26;333(7565):448. doi: 10.1136/bmj.333.7565.448-a. BMJ. 2006. PMID: 16931855 Free PMC article. No abstract available.
References
-
- Fortney JT, Gan TJ, Graczyk S, Wetchler B, Melson T, Khalil S, et al. A comparison of the efficacy, safety, and patient satisfaction of ondansetron versus droperidol as antiemetics for elective outpatient surgical procedures. Anesth Analg 1998;86: 731-8. - PubMed
-
- Koivuranta M, Läärä E, Snåre L, Alahuhta S. A survey of postoperative nausea and vomiting. Anaesthesia 1997;52: 443-9. - PubMed
-
- Wang JJ, Ho ST, Lee SC, Liu YC, Liu YH, Liao YC. The prophylactic effect of dexamethasone on postoperative nausea and vomiting in women undergoing thyroidectomy: a comparison of droperidol with saline. Anesth Analg 1999;89: 200-3. - PubMed
-
- Henzi I, Walder B, Tramer MR. Metoclopramide in the prevention of postoperative nausea and vomiting: a quantitative systematic review of randomized, placebo-controlled studies. Br J Anaesth 1999;83: 761-71. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources