Regulation of neddylation and deneddylation of cullin1 in SCFSkp2 ubiquitin ligase by F-box protein and substrate
- PMID: 16861300
- PMCID: PMC1544201
- DOI: 10.1073/pnas.0603921103
Regulation of neddylation and deneddylation of cullin1 in SCFSkp2 ubiquitin ligase by F-box protein and substrate
Abstract
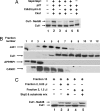
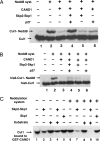
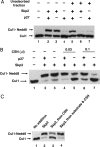
The activity of cullin-containing ubiquitin protein ligase complexes is stimulated by linkage to cullin of the ubiquitin-like protein Nedd8 ("neddylation"). Neddylation is inhibited by the tight binding of cullins to CAND1 (cullin-associated and neddylation-dissociated 1) protein, and Nedd8 is removed from cullins by specific isopeptidase activity of the COP9/signalosome (CSN) complex. The mechanisms that regulate neddylation and deneddylation of cullins were unknown. We examined this problem for the case of SCF(Skp2), a cullin1 (Cul1)-containing ubiquitin ligase complex that contains the S phase-associated protein Skp2 as the substrate-binding F-box protein subunit. SCF(Skp2) targets for degradation the cyclin-dependent kinase (cdk) inhibitor p27 in the G(1)-to-S phase transition, a process that requires its phosphorylation and binding to cdk2-cyclin E. Because levels of Skp2, cyclin E, and the accessory protein Cks1 (cyclin kinase subunit 1) all rise at the end of G(1) phase, it seemed possible that the neddylation of Cul1 in SCF(Skp2) is regulated by the availability of the F-box protein and/or the substrate. We found that the supplementation of Skp2-Skp1 and substrate (along with further components necessary for substrate presentation to the ubiquitin ligase) to extracts of HeLa cells synergistically increased levels of neddylated Cul1. Skp2-Skp1 abrogates the inhibitory influence of CAND1 on the neddylation of Cul1 by promoting the dissociation of the cullin-CAND1 complex, whereas substrate, together with substrate-presenting components, prevents the action of CSN to deneddylate cullin. We propose a sequence of events in which the increased availability of Skp2 and substrate in the transition of cells to S phase promotes the neddylation and assembly of the SCF(Skp2) ubiquitin ligase complex.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
References
-
- Petroski M. D., Deshaies R. J. Nat. Rev. Mol. Cell Biol. 2005;6:9–20. - PubMed
-
- Cardozo T., Pagano M. Nat. Rev. Mol. Cell Biol. 2004;5:739–751. - PubMed
-
- Carrano A. C., Eytan E., Hershko A., Pagano M. Nat. Cell Biol. 1999;1:193–199. - PubMed
-
- Ganoth D., Bornstein G., Ko T. K., Larsen B., Tyers M., Pagano M., Hershko A. Nat. Cell Biol. 2001;3:321–324. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

