Genomic organization, differential expression, and interaction of SQUAMOSA promoter-binding-like transcription factors and microRNA156 in rice
- PMID: 16861571
- PMCID: PMC1557610
- DOI: 10.1104/pp.106.084475
Genomic organization, differential expression, and interaction of SQUAMOSA promoter-binding-like transcription factors and microRNA156 in rice
Abstract
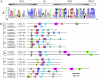
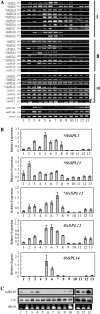
Transcription factors play essential roles in the developmental processes of plants. Many such factors are regulated by microRNAs (miRNAs). SQUAMOSA (SQUA) promoter-binding-like (SPL) genes encode plant-specific transcription factors, some of which contain complementary sequences of miRNA156. In this study, 19 rice (Oryza sativa) SPL (OsSPL) genes and 12 rice miRNA156 (OsmiR156) precursors were identified in the rice genome. Sequence and experimental analysis suggested that 11 OsSPL genes were putative targets of OsmiR156. Plant SPL proteins were classified into six subgroups based on the phylogenetic analysis of SQUA promoter-binding protein domain. Diverse exon-intron structures and distinct organizations of putative motifs beyond the SQUA promoter-binding protein domains were identified in the OsSPL gene family. Transcript level analysis of OsSPL genes in various rice tissues and organs revealed different tempospatial expression patterns. More than half of the OsSPL genes including most OsmiR156-targeted genes are predominantly expressed in the young panicles, whereas OsmiR156 genes are predominantly expressed in the young shoots and leaves of rice. Overexpression of two OsmiR156 genes (OsmiR156b and OsmiR156h) in rice resulted in severe dwarfism, strongly reduced panicle size, and delayed flowering, suggesting that OsmiR156 and OsSPL target genes are involved in various developmental processes, especially the flower development of rice. Different patterns of transcript changes (decreased or unchanged) of different target genes in same tissue and of same target gene in different tissues detected in the OsmiR156-overexpressing plants suggested diverse interactions between OsmiR156 and OsSPL target genes in a tissue-specific manner.
Figures




References
-
- Allen E, Xie Z, Gustafson AM, Sung GH, Spatafora JW, Carrington JC (2004) Evolution of microRNA genes by inverted duplication of target gene sequences in Arabidopsis thaliana. Nat Genet 36: 1282–1290 - PubMed
-
- Arazi T, Talmor-Neiman M, Stav R, Riese M, Huijser P, Baulcombe DC (2005) Cloning and characterization of micro-RNAs from moss. Plant J 43: 837–848 - PubMed
-
- Bae KH, Kwon YD, Shin HC, Hwang MS, Ryu EH, Park KS, Yang HY, Lee DK, Lee Y, Park J, et al (2003) Human zinc fingers as building blocks in the construction of artificial transcription factors. Nat Biotechnol 21: 275–280 - PubMed
-
- Bailey TL, Elkan C (1994) Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc Int Conf Intell Syst Mol Biol 2: 28–36 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

