Expression and antimicrobial function of bactericidal permeability-increasing protein in cystic fibrosis patients
- PMID: 16861658
- PMCID: PMC1539578
- DOI: 10.1128/IAI.02066-05
Expression and antimicrobial function of bactericidal permeability-increasing protein in cystic fibrosis patients
Abstract
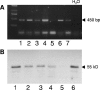
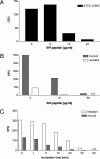
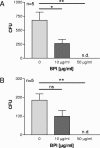
In cystic fibrosis (CF), the condition limiting the prognosis of affected children is the chronic obstructive lung disease accompanied by chronic and persistent infection with mostly mucoid strains of Pseudomonas aeruginosa. The majority of CF patients have antineutrophil cytoplasmic antibodies (ANCA) primarily directed against the bactericidal permeability-increasing protein (BPI) potentially interfering with antimicrobial effects of BPI. We analyzed the expression of BPI in the airways of patients with CF. In their sputum samples or bronchoalveolar lavage specimens, nearly all patients expressed BPI mRNA and protein, which were mainly products of neutrophil granulocytes as revealed by intracellular staining and subsequent flow cytometry. Repeated measurements revealed consistent individual BPI expression levels during several months quantitatively correlating with interleukin-8. In vitro, P. aeruginosa isolates from CF patients initiated the rapid release of BPI occurring independently of protein de novo syntheses. Furthermore, purified natural BPI as well as a 27-mer BPI-derived peptide displayed antimicrobial activity against even patient-derived mucoid P. aeruginosa strains and bacteria resistant against all antibiotics tested. Thus, BPI that is functionally active against mucoid P. aeruginosa strains is expressed in the airways of CF patients but may be hampered by autoantibodies, resulting in chronic infection.
Figures


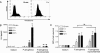
References
-
- Aebi, C., F. Theiler, C. C. Aebischer, and M. H. Schoeni. 2000. Autoantibodies directed against bactericidal/permeability-increasing protein in patients with cystic fibrosis: association with microbial respiratory tract colonization. Pediatr. Infect. Dis. J. 19:207-212. - PubMed
-
- Calafat, J., H. Janssen, E. F. Knol, J. Malm, and A. Egesten. 2000. The bactericidal/permeability-increasing protein (BPI) is membrane-associated in azurophil granules of human neutrophils, and relocation occurs upon cellular activation. APMIS 108:201-208. - PubMed
-
- Carlsson, M., L. Eriksson, I. Erwander, J. Wieslander, and M. Segelmark. 2003. Pseudomonas-induced lung damage in cystic fibrosis correlates to bactericidal-permeability increasing protein (BPI)-autoantibodies. Clin. Exp. Rheumatol. 21:S95-100. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

