ATR-dependent checkpoint modulates XPA nuclear import in response to UV irradiation
- PMID: 16862173
- PMCID: PMC3106104
- DOI: 10.1038/sj.onc.1209828
ATR-dependent checkpoint modulates XPA nuclear import in response to UV irradiation
Abstract
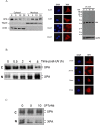
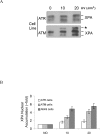
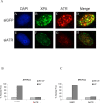
In response to DNA damage, mammalian cells activate various DNA repair pathways to remove DNA lesions and, meanwhile, halt cell cycle progressions to allow sufficient time for repair. The nucleotide excision repair (NER) and the ATR-dependent cell cycle checkpoint activation are two major cellular responses to DNA damage induced by UV irradiation. However, how these two processes are coordinated in the response is poorly understood. Here we showed that the essential NER factor XPA (xeroderma pigmentosum group A) underwent nuclear accumulation upon UV irradiation, and strikingly, such an event occurred in an ATR (Ataxia-Telangiectasia mutated and RAD3-related)-dependent manner. Either treatment of cells with ATR kinase inhibitors or transfection of cells with small interfering RNA targeting ATR compromised the UV-induced XPA nuclear translocation. Consistently, the ATR-deficient cells displayed no substantial XPA nuclear translocation while the translocation remained intact in ATM (Ataxia-Telangiectasia mutated)-deficient cells in response to UV irradiation. Moreover, we found that ATR is required for the UV-induced nuclear focus formation of XPA. Taken together, our results suggested that the ATR checkpoint pathway may modulate NER activity through the regulation of XPA redistribution in human cells upon UV irradiation.
Figures


References
-
- Abraham RT. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 2001;15:2177–96. - PubMed
-
- Barr SM, Leung CG, Chang EE, Cimprich KA. ATR kinase activity regulates the intranuclear translocation of ATR and RPA following ionizing radiation. Curr Biol. 2003;13:1047–51. - PubMed
-
- Bartek J, Lukas C, Lukas J. Checking on DNA damage in S phase. Nat Rev Mol Cell Biol. 2004;5:792–804. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

