To stabilize neutrophil polarity, PIP3 and Cdc42 augment RhoA activity at the back as well as signals at the front
- PMID: 16864657
- PMCID: PMC2064239
- DOI: 10.1083/jcb.200604113
To stabilize neutrophil polarity, PIP3 and Cdc42 augment RhoA activity at the back as well as signals at the front
Abstract
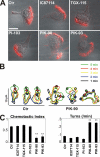
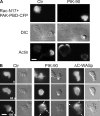
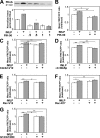
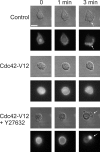
Chemoattractants like f-Met-Leu-Phe (fMLP) induce neutrophils to polarize by triggering divergent signals that promote the formation of protrusive filamentous actin (F-actin; frontness) and RhoA-dependent actomyosin contraction (backness). Frontness locally inhibits backness and vice versa. In neutrophil-like HL60 cells, blocking phosphatidylinositol-3,4,5-tris-phosphate (PIP3) accumulation with selective inhibitors of PIP3 synthesis completely prevents fMLP from activating a PIP3-dependent kinase and Cdc42 but not from stimulating F-actin accumulation. PIP3-deficient cells show reduced fMLP-dependent Rac activity and unstable pseudopods, which is consistent with the established role of PIP3 as a mediator of positive feedback pathways that augment Rac activation at the front. Surprisingly, such cells also show reduced RhoA activation and RhoA-dependent contraction at the trailing edge, leading to the formation of multiple lateral pseudopods. Cdc42 mediates PIP3's positive effect on RhoA activity. Thus, PIP3 and Cdc42 maintain stable polarity with a single front and a single back not only by strengthening pseudopods but also, at longer range, by promoting RhoA-dependent actomyosin contraction at the trailing edge.
Figures

Similar articles
-
Neutrophil polarization: spatiotemporal dynamics of RhoA activity support a self-organizing mechanism.Proc Natl Acad Sci U S A. 2006 Mar 7;103(10):3639-44. doi: 10.1073/pnas.0600092103. Epub 2006 Feb 28. Proc Natl Acad Sci U S A. 2006. PMID: 16537448 Free PMC article.
-
Rac and Cdc42 play distinct roles in regulating PI(3,4,5)P3 and polarity during neutrophil chemotaxis.J Cell Biol. 2003 Feb 3;160(3):375-85. doi: 10.1083/jcb.200208179. Epub 2003 Jan 27. J Cell Biol. 2003. PMID: 12551955 Free PMC article.
-
Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils.Cell. 2003 Jul 25;114(2):201-14. doi: 10.1016/s0092-8674(03)00555-5. Cell. 2003. PMID: 12887922
-
[Transport of PIP3 mediated by kinesin regulates neuronal cell polarity].Tanpakushitsu Kakusan Koso. 2007 Jul;52(8):893-9. Tanpakushitsu Kakusan Koso. 2007. PMID: 17642273 Review. Japanese. No abstract available.
-
Establishment and maintenance of cell polarity during leukocyte chemotaxis.Cell Adh Migr. 2007 Apr-Jun;1(2):69-76. doi: 10.4161/cam.1.2.4547. Epub 2007 Apr 6. Cell Adh Migr. 2007. PMID: 19329880 Free PMC article. Review.
Cited by
-
Bound attractant at the leading vs. the trailing edge determines chemotactic prowess.Proc Natl Acad Sci U S A. 2007 Aug 14;104(33):13349-54. doi: 10.1073/pnas.0705889104. Epub 2007 Aug 7. Proc Natl Acad Sci U S A. 2007. PMID: 17684096 Free PMC article.
-
The role of PI3Kγ in the immune system: new insights and translational implications.Nat Rev Immunol. 2022 Nov;22(11):687-700. doi: 10.1038/s41577-022-00701-8. Epub 2022 Mar 23. Nat Rev Immunol. 2022. PMID: 35322259 Free PMC article. Review.
-
The role of polarisation of circulating tumour cells in cancer metastasis.Cell Mol Life Sci. 2019 Oct;76(19):3765-3781. doi: 10.1007/s00018-019-03169-3. Epub 2019 Jun 19. Cell Mol Life Sci. 2019. PMID: 31218452 Free PMC article. Review.
-
Mammalian target of rapamycin and Rictor control neutrophil chemotaxis by regulating Rac/Cdc42 activity and the actin cytoskeleton.Mol Biol Cell. 2013 Nov;24(21):3369-80. doi: 10.1091/mbc.E13-07-0405. Epub 2013 Sep 4. Mol Biol Cell. 2013. PMID: 24006489 Free PMC article.
-
Subcellular curvature at the perimeter of micropatterned cells influences lamellipodial distribution and cell polarity.Cell Motil Cytoskeleton. 2008 Nov;65(11):841-52. doi: 10.1002/cm.20305. Cell Motil Cytoskeleton. 2008. PMID: 18677773 Free PMC article.
References
-
- Bokoch, G.M. 2003. Biology of the p21-activated kinases. Annu. Rev. Biochem. 72:743–781. - PubMed
-
- Chung, C.Y., G. Potikyan, and R.A. Firtel. 2001. Control of cell polarity and chemotaxis by Akt/PKB and PI3 kinase through the regulation of PAKa. Mol. Cell. 7:937–947. - PubMed
-
- de Anda, F.C., G. Pollarolo, J.S. Da Silva, P.G. Camoletto, F. Feiguin, and C.G. Dotti. 2005. Centrosome localization determines neuronal polarity. Nature. 436:704–708. - PubMed

