Properties regulating the nature of the plasmacytoid dendritic cell response to Toll-like receptor 9 activation
- PMID: 16864658
- PMCID: PMC2118381
- DOI: 10.1084/jem.20060401
Properties regulating the nature of the plasmacytoid dendritic cell response to Toll-like receptor 9 activation
Abstract
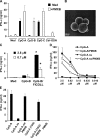
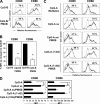
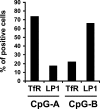
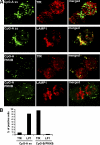
Human plasmacytoid dendritic cells (PDCs) can produce interferon (IFN)-alpha and/or mature and participate in the adaptive immune response. Three classes of CpG oligonucleotide ligands for Toll-like receptor (TLR)9 can be distinguished by different sequence motifs and different abilities to stimulate IFN-alpha production and maturation of PDCs. We show that the nature of the PDC response is determined by the higher order structure and endosomal location of the CpG oligonucleotide. Activation of TLR9 by the multimeric CpG-A occurs in transferrin receptor (TfR)-positive endosomes and leads exclusively to IFN-alpha production, whereas monomeric CpG-B oligonucleotides localize to lysosome-associated membrane protein (LAMP)-1-positive endosomes and promote maturation of PDCs. However, CpG-B, when complexed into microparticles, localizes in TfR-positive endosomes and induces IFN-alpha from PDCs, whereas monomeric forms of CpG-A localize to LAMP-1-positive endosomes accompanied by the loss of IFN-alpha production and a gain in PDC maturation activity. CpG-C sequences, which induce both IFN-alpha and maturation of PDCs, are distributed in both type of endosomes. Encapsulation of CpG-C in liposomes stable above pH 5.75 completely abrogated the IFN-alpha response while increasing PDC maturation. This establishes that the primary determinant of TLR9 signaling is not valency but endosomal location and demonstrates a strict compartmentalization of the biological response to TLR9 activation in PDCs.
Figures



References
-
- Liu, Y.J. 2005. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 23:275–306. - PubMed
-
- Svensson, H., B. Cederblad, M. Lindahl, and G. Alm. 1996. Stimulation of natural interferon-α/β-producing cells by Staphylococcus aureus. J. Interferon Cytokine Res. 16:7–16. - PubMed
-
- Cella, M., D. Jarrossay, F. Facchetti, O. Alebardi, H. Nakajima, A. Lanzavecchia, and M. Colonna. 1999. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 5:919–923. - PubMed
-
- Siegal, F.P., N. Kadowaki, M. Shodell, P.A. Fitzgerald-Bocarsly, K. Shah, S. Ho, S. Antonenko, and Y.J. Liu. 1999. The nature of the principal type 1 interferon-producing cells in human blood. Science. 284:1835–1837. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

