Glatiramer acetate fights against Alzheimer's disease by inducing dendritic-like microglia expressing insulin-like growth factor 1
- PMID: 16864778
- PMCID: PMC1544247
- DOI: 10.1073/pnas.0604681103
Glatiramer acetate fights against Alzheimer's disease by inducing dendritic-like microglia expressing insulin-like growth factor 1
Abstract
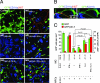
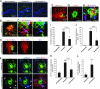
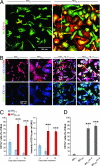
Alzheimer's disease (AD) is characterized by plaque formation, neuronal loss, and cognitive decline. The functions of the local and systemic immune response in this disease are still controversial. Using AD double-transgenic (APP/PS1) mice, we show that a T cell-based vaccination with glatiramer acetate, given according to a specific regimen, resulted in decreased plaque formation and induction of neurogenesis. It also reduced cognitive decline, assessed by performance in a Morris water maze. The vaccination apparently exerted its effect by causing a phenotype switch in brain microglia to dendritic-like (CD11c) cells producing insulin-like growth factor 1. In vitro findings showed that microglia activated by aggregated beta-amyloid, and characterized as CD11b(+)/CD11c(-)/MHC class II(-)/TNF-alpha(+) cells, impeded neurogenesis from adult neural stem/progenitor cells, whereas CD11b(+)/CD11c(+)/MHC class II(+)/TNF-alpha(-) microglia, a phenotype induced by IL-4, counteracted the adverse beta-amyloid-induced effect. These results suggest that dendritic-like microglia, by facilitating the necessary adjustment, might contribute significantly to the brain's resistance to AD and argue against the use of antiinflammatory drugs.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

