CDC7 kinase phosphorylates serine residues adjacent to acidic amino acids in the minichromosome maintenance 2 protein
- PMID: 16864800
- PMCID: PMC1544202
- DOI: 10.1073/pnas.0604990103
CDC7 kinase phosphorylates serine residues adjacent to acidic amino acids in the minichromosome maintenance 2 protein
Abstract
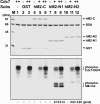
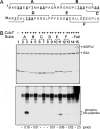
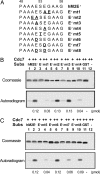
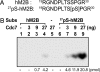
Cdc7 is an essential kinase required for the initiation of eukaryotic DNA replication. Previous studies in many species showed that the minichromosome maintenance complex is a major physiological target of this kinase. In this study, we have mapped the sites in human Mcm2 protein that are phosphorylated by Cdc7. The in vitro phosphorylation of several Mcm2 truncated proteins and peptides revealed that Mcm2 contains two major ((5)S and (53)S) and at least three minor phosphorylation sites ((4)S, (7)S, and (59)T) located at the N-terminal region. Alanine substitution experiments with Mcm2 peptides showed that the phosphorylation of (5)S and (53)S by Cdc7 required the presence of an acidic amino acid adjacent to a serine residue. Furthermore, although Cdc7 was unable to phosphorylate a Mcm2 peptide (spanning amino acids 19-30 and containing (26)S and (27)S), it phosphorylated (26)S efficiently when this peptide contained a chemically synthesized phospho-(27)S modification. Hence, additional Cdc7 phosphorylation sites could be generated in Mcm2 by its prior phosphorylation by a cyclin-dependent kinase. This finding may explain why the sequential action of cyclin-dependent and Cdc7 kinases is essential for the initiation of DNA replication.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

